Assertion : Al2O3 is converted into Al by reduction with carbon at high temp.
Reason : Carbon has greater affinity for oxygen than aluminium.
If both assetion and reason are CORRECT, and reason is the CORRECT explanation of the assertion
If both assertion and reason are CORRECT, but reason is NOT the CORRECT explanation of the assertion
If assertion is CORRECT but reason is INCORRECT
If assertion is INCORRECT but reason is CORRECT
The correct Answer is:C
To solve the question, we need to analyze the assertion and the reason provided:
Assertion: Al₂O₃ is converted into Al by reduction with carbon at high temperature.
Reason: Carbon has greater affinity for oxygen than aluminum.
1. Understanding the Assertion:
- The assertion states that aluminum oxide (Al₂O₃) can be reduced to aluminum (Al) using carbon as a reducing agent at high temperatures. This is a common method used in metallurgy, particularly in the extraction of aluminum.
2. Analyzing the Reduction Process:
- In the reduction process, a reducing agent donates electrons to the metal oxide, resulting in the formation of the metal and the oxide of the reducing agent. In this case, carbon can reduce Al₂O₃ to aluminum, but it requires high temperatures to do so.
3. Examining the Reason:
- The reason states that carbon has a greater affinity for oxygen than aluminum. This implies that carbon would prefer to bond with oxygen rather than aluminum would. However, this statement is incorrect in the context of the thermite reaction, where aluminum is actually a stronger reducing agent than carbon.
4. Understanding Affinity for Oxygen:
- Aluminum has a very high affinity for oxygen, which means it readily reacts with oxygen to form Al₂O₃. In fact, aluminum is often used as a reducing agent because it can displace other metals from their oxides due to its strong affinity for oxygen.
5. Conclusion:
- The assertion is correct because Al₂O₃ can indeed be reduced to aluminum using carbon at high temperatures. However, the reason is incorrect because it misrepresents the relative affinities of carbon and aluminum for oxygen. Therefore, the correct answer is that the assertion is true, but the reason is false.
Final Answer:
- Assertion is correct, Reason is incorrect.
Reason: Carbon has greater affinity for oxygen than aluminum.
- The assertion states that aluminum oxide (Al₂O₃) can be reduced to aluminum (Al) using carbon as a reducing agent at high temperatures. This is a common method used in metallurgy, particularly in the extraction of aluminum.
- In the reduction process, a reducing agent donates electrons to the metal oxide, resulting in the formation of the metal and the oxide of the reducing agent. In this case, carbon can reduce Al₂O₃ to aluminum, but it requires high temperatures to do so.
- The reason states that carbon has a greater affinity for oxygen than aluminum. This implies that carbon would prefer to bond with oxygen rather than aluminum would. However, this statement is incorrect in the context of the thermite reaction, where aluminum is actually a stronger reducing agent than carbon.
- Aluminum has a very high affinity for oxygen, which means it readily reacts with oxygen to form Al₂O₃. In fact, aluminum is often used as a reducing agent because it can displace other metals from their oxides due to its strong affinity for oxygen.
- The assertion is correct because Al₂O₃ can indeed be reduced to aluminum using carbon at high temperatures. However, the reason is incorrect because it misrepresents the relative affinities of carbon and aluminum for oxygen. Therefore, the correct answer is that the assertion is true, but the reason is false.
- Assertion is correct, Reason is incorrect.
Topper's Solved these Questions
- METALLURGYBOOK - VK JAISWAL ENGLISHCHAPTER - METALLURGYEXERCISE - MATCH THE COLUMN
20 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - VK JAISWAL ENGLISHCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - ASSERTION-REASON TYPE QUESTIONS
14 Videos
- p-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - p-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
35 Videos
Similar Questions
Column-I and Column-II contains four entries each. Entries of column-I are to be matched with some entries of column-II. Each entry of column-I may have the matching with one or more than one entries of column-II.
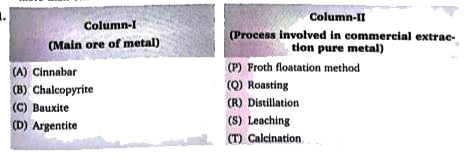
View SolutionAssertion (A) Green house effect was observed in houses used to grow plants and these are made of green glass.
Reason (R) Green house name has been given because glass houses are made of green glass.
View SolutionIn phosphorus acid, If X is number of non-bonding electron pairs. Y is number of σ-bonds and Z is number of π-bonds. Then calculate value of "YxZ-X".
View SolutionAssertion : Al2O3 is converted into Al by reduction with carbon at high temp.
Reason : Carbon has greater affinity for oxygen than aluminium.
View SolutionAssertion : Al2O3 is converted into Al by reduction with carbon at high temp.
Reason : Carbon has greater affinity for oxygen than aluminium.
View SolutionAssertion : Al_2O_3 is converted to aluminium by reduction with carbon. Reason : Carbon (graphite) has greater affinity for oxygen than Al.
View SolutionAssertion : Al2O3 is converted into Al by reduction with carbon at high temp.
Reason : Carbon has greater affinity for oxygen than aluminium.
View SolutionAssertion. Al is obtained by high temperature reduction of alumina with carbon. Reason. Alumina reacts with carbon to form aluminium carbide which decomposes at high temperature to from Al while carbon is oxidised to CO.
View SolutionAssertion : Al2O3 is converted into Al by reduction with carbon at high temp.
Reason : Carbon has greater affinity for oxygen than aluminium.
View SolutionCarbon anode in the electrolytic reduction of Al2O3.
View SolutionAssertion : Al_(2)O_(3) is converted into Al by reduction with carbon at high temp. Reason : Carbon has greater affinity for oxygen than aluminium.
View SolutionAl2O3 का अपचयन कार्बन के द्वारा सम्भव नहीं हैं क्योंकि
View SolutionRead the following statements :- (I) Al has greater affinity than that of Cr, for oxygen (II) Al can be used for reduction of Fe_(2)O_(3) (III) Carbon is used for reduction of copper oxide (CuO) (IV) SnO_(2) can be reduced by carbon Choose the correct set of statement(s)
View SolutionAssertion : Al2O3 is converted into Al by reduction with carbon at high temp.
Reason : Carbon has greater affinity for oxygen than aluminium.
View SolutionCarbon cannot be used in the reduction of Al2O3 because
View Solution

