(NH4)2Cr2O7 on heating liberates a gas. The same gas will be obtained by
Heating NH4NO2
Heating NH4NO3
Heating (NH4)2SO4
Treatment Mg3N2 with H2O
The correct Answer is:A
To solve the question regarding the gas liberated when (NH4)2Cr2O7 (ammonium dichromate) is heated, we will follow these steps:
Step 1: Identify the reaction of ammonium dichromate on heating
When ammonium dichromate is heated, it decomposes to produce nitrogen gas, water vapor, and chromium(III) oxide. The balanced equation for this reaction is:
(NH4)2Cr2O7→Cr2O3+N2+4H2O
Step 2: Determine the gas produced
From the decomposition reaction, we can see that the gas produced is nitrogen gas (N₂).
Step 3: Analyze the options given in the question
We need to check which of the given options also produces nitrogen gas when heated:
1. Heating NH4NO2 (ammonium nitrite):
NH4NO2→N2+2H2O
This reaction produces nitrogen gas.
2. Heating NH4NO3 (ammonium nitrate):
NH4NO3→N2O+2H2O
This reaction produces nitrous oxide (N₂O), not nitrogen gas.
3. Heating (NH4)2SO4 (ammonium sulfate):
(NH4)2SO4→2NH3+H2SO4
This reaction produces ammonia gas (NH₃) and sulfuric acid (H₂SO₄), not nitrogen gas.
4. Treatment of Mg3N2 with H2O (magnesium nitride with water):
Mg3N2+6H2O→3Mg(OH)2+2NH3
This reaction produces ammonia gas, not nitrogen gas.
Step 4: Conclusion
From the analysis, the only option that produces nitrogen gas is the first option, heating ammonium nitrite (NH4NO2). Therefore, the answer to the question is:
The same gas (nitrogen gas) will be obtained by heating NH4NO2.
---
When ammonium dichromate is heated, it decomposes to produce nitrogen gas, water vapor, and chromium(III) oxide. The balanced equation for this reaction is:
From the decomposition reaction, we can see that the gas produced is nitrogen gas (N₂).
We need to check which of the given options also produces nitrogen gas when heated:
NH4NO2→N2+2H2O
This reaction produces nitrogen gas.
NH4NO3→N2O+2H2O
This reaction produces nitrous oxide (N₂O), not nitrogen gas.
(NH4)2SO4→2NH3+H2SO4
This reaction produces ammonia gas (NH₃) and sulfuric acid (H₂SO₄), not nitrogen gas.
Mg3N2+6H2O→3Mg(OH)2+2NH3
This reaction produces ammonia gas, not nitrogen gas.
From the analysis, the only option that produces nitrogen gas is the first option, heating ammonium nitrite (NH4NO2). Therefore, the answer to the question is:
টপার এই প্রশ্নগুলো সমাধান করেছে
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - LEVEL 3
36 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - ONE OR MORE ANSWERS IS/ARE CORRECT
40 Videos
- TYPES OF REACTIONSBOOK - VK JAISWAL ENGLISHCHAPTER - TYPES OF REACTIONSEXERCISE - MATCH THE COLUMN
3 Videos
- s-BLOCK ELEMENTSBOOK - VK JAISWAL ENGLISHCHAPTER - s-BLOCK ELEMENTSEXERCISE - SUBJECTIVE PROBLEMS
2 Videos
সম্পর্কিত প্রশ্ন
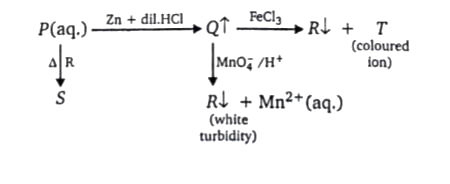
Q. Species P and S are respectively:
View SolutionWhich of the following combination of species can evolve O2?
View SolutionMatch the following columns

View Solution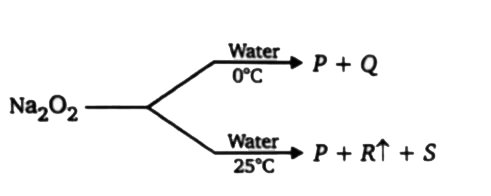
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionWhen (NH4)2Cr2O7 is heated, the gas evolved is
View Solution(NH_(4))_(2)Cr_(2)O_(7) on heating gives a gas which is also given by :
View Solution(NH_(4))_(2)Cr_(2)O_(7) on heating gives a gas which is also given by
View Solution(NH_(4))_(2)Cr_(2)O_(7) on heating liberates a gas. The same gas will be obtained by
View SolutionName the gas evolved when ammonium dichromate (NH_(4))_(2)Cr_(2)O_(7) is heated.
View Solution(NH_(4))_(2)Cr_(2)O_(7) overset("heat") to
View SolutionWhen (NH_(4))_(2)Cr_(2)O_(7) is heated
View SolutionOn heating K_(2)Cr_(2)O_(7) with NaCl and conc. H_(2)SO_(4) , the gas liberated is
View Solution(NH_(4))_(2)Cr_(2)O_(7) on heating gives
View Solution(NH4)2Cr2O7 को गर्म करने पर एक गैस प्राप्त होती है, जिसे निम्न के द्वारा भी प्राप्त किया जा सकता है
View Solution(NH4)2Cr2O7 on heating liberates a gas. The same gas will be obtained by
View Solutionजब (NH4)2Cr2O7 को गर्म किया जाता है तो कौन सी गैस निकलती है?
View Solution

