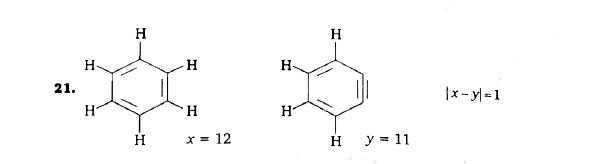
Calculate value of |x-y|, here x and y are the total number of bonds in benzene and benzyne respectively which are formed by overlapping of hybridized orbitals.
The correct Answer is:1
Topper's Solved these Questions
- CHEMICAL BONDING (BASIC)BOOK - VK JAISWAL ENGLISHCHAPTER - CHEMICAL BONDING (BASIC)EXERCISE - ASSERTION-REASON TYPE QUESTIONS
23 Videos
- CHEMICAL BONDING (ADVANCED)BOOK - VK JAISWAL ENGLISHCHAPTER - CHEMICAL BONDING (ADVANCED)EXERCISE - SUBJECTIVE PROBLEMS
48 Videos
- CO-ORDINATION COMPOUNDSBOOK - VK JAISWAL ENGLISHCHAPTER - CO-ORDINATION COMPOUNDSEXERCISE - SUBJECTIVE PROBLEMS
42 Videos
Similar Questions
Assertion : Multiple bond between two bonded atoms can have more than three bonds.
Reason : Multiple bond between two bonded atoms can not have more than two π-bonds.
View SolutionThere are two groups of compounds A and B. Groups A contains three compounds Px4,Qy3,Rz2. Groups B also contains three compounds Sx4,Ty3,Uz2. Hybridization of each central atom of group A compounds is same as that of iodine in IBrCl− while in group B compounds it is same as that of iodine IBrCl+. Substituents X, Y and Z exhibit covalency of one in ground state. Then find the value of x/y.
Where, x and y are total number of lone pair present at central atoms of compounds of group A and B respectively.
View SolutionConsider the following compounds with their regular geometries.

Calculate value of (x+y−z)2, where x is total number of axial d-orbitals having zero nodal plane, y and z are total no. of non-axial and axial d-orbitals respectively each one having two nodal planes used in hybridisation of central atoms of compounds A to E.
View SolutionWhat orbitals can overlap to form a sigma -bond and which orbitals can overlap to form a pi -bond?
View Solutionp_(y) -orbital can not form pi -bond by lateral overlap with :
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (pi) bond(s) and sigma (sigma) bonds in hydrogen phosphite ion respectively.
View SolutionCalculate value of |x-y|, here x and y are the total number of bonds in benzene and benzyne respectively which are formed by overlapping of hybridized orbitals.
View Solutionpy-orbital can not form π -bond by lateral overlap with :
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View SolutionCalculate value of |x-y|, here x and y are the total number of bonds in benzene and benzyne respectively which are formed by overlapping of hybridized orbitals.
View SolutionCalculate value of |x-y| , here x and y are the total number of bonds in benzene and benyne respectively which are formed by overlapping of hybridized orbitals .
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View SolutionWhich bond is formed by overlapping of 2px with 2py? Why ?
View SolutionA bonds are formed by the ______ overlap of ______ orbitals:
View Solutionp_(y) -orbital can not form pi -bond by lateral overlap with :
View SolutionCalculate the value of " x+y-z" here x,y and z are total number of non-bonded electron pair (s),pie (π) bond(s) and sigma (σ) bonds in hydrogen phosphite ion respectively.
View Solution