Among the following, which has the lowest enthalpy of fussion?
Flourine
Hydrogen
Chlorine
Helium
The correct Answer is:D
To determine which substance has the lowest enthalpy of fusion among the given options, we need to understand the concept of enthalpy of fusion and the factors that influence it.
1. Understanding Enthalpy of Fusion:
- Enthalpy of fusion (also known as latent heat of fusion) is the amount of energy required to change a substance from a solid to a liquid at constant pressure. This process involves breaking intermolecular forces in the solid state.
2. Factors Affecting Enthalpy of Fusion:
- The enthalpy of fusion is influenced by the strength of the intermolecular forces holding the solid together. Substances with weaker intermolecular forces generally have lower enthalpy of fusion, as less energy is needed to overcome these forces.
3. Comparison of Substances:
- To find the substance with the lowest enthalpy of fusion, we can compare the enthalpy values of different substances. In this case, we are particularly interested in helium, which is known for its unique properties.
4. Helium’s Unique Properties:
- Helium is a noble gas with a very low boiling point and enthalpy of fusion. It has a complete electron configuration (1s²), and its atoms are held together by very weak van der Waals forces. This means that it requires very little energy to change from solid to liquid.
5. Conclusion:
- Among the substances considered, helium has the lowest enthalpy of fusion due to its weak intermolecular forces and unique atomic structure. Therefore, the answer is helium.
Final Answer:
Helium has the lowest enthalpy of fusion among the given options.
---
- Enthalpy of fusion (also known as latent heat of fusion) is the amount of energy required to change a substance from a solid to a liquid at constant pressure. This process involves breaking intermolecular forces in the solid state.
- The enthalpy of fusion is influenced by the strength of the intermolecular forces holding the solid together. Substances with weaker intermolecular forces generally have lower enthalpy of fusion, as less energy is needed to overcome these forces.
- To find the substance with the lowest enthalpy of fusion, we can compare the enthalpy values of different substances. In this case, we are particularly interested in helium, which is known for its unique properties.
- Helium is a noble gas with a very low boiling point and enthalpy of fusion. It has a complete electron configuration (1s²), and its atoms are held together by very weak van der Waals forces. This means that it requires very little energy to change from solid to liquid.
- Among the substances considered, helium has the lowest enthalpy of fusion due to its weak intermolecular forces and unique atomic structure. Therefore, the answer is helium.
Helium has the lowest enthalpy of fusion among the given options.
টপার এই প্রশ্নগুলো সমাধান করেছে
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (ADVANCED)Exercise:Level 2
Explore 143 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (ADVANCED)Exercise:Level 3
Explore 72 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWAL ENGLISHChapter:CHEMICAL BONDING (BASIC)Exercise:SUBJECTIVE PROBLEMS
Explore 49 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Which of the following has the lowest heat of combustion?
A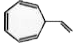 B
B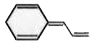 C
C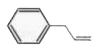 D
D
Among the following sweeteners which one has the lowest sweetness value?
AAlitameBAspartameCSaccharinDSucralose
Which of the following has the lowest density?
View SolutionWhich one has the lowest value
View SolutionAmong the following, the solution which shows the lowest osmotic pressure is:
View SolutionWhich has lowest wavelength :-
View SolutionWhich of the following compounds has a positive enthalpy of solution?
View SolutionWhich of the following acid has the lowest value of acid dissociation constant :
View Solution
