A stationary cylinder of oxygent used in a hospital has the following characteristics at room temperature 300K, gauge pressure 1.38×107 Pa. volume 16L. If the flow area, measured at atmospheric pressure, is constant at 2.4L/min, the cylinder will last for nearly
5h
10h
15h
20h
The correct Answer is:C
To solve the problem step by step, we will use the ideal gas law and the information provided about the cylinder of oxygen.
Step 1: Convert the volume of the cylinder from liters to cubic meters.
Given:
- Volume V1=16L=16×10−3m3
Step 2: Identify the initial conditions.
- Initial temperature T=300K
- Initial pressure P1=1.38×107Pa
- Final pressure P2=Patm=105Pa
Step 3: Set up the equation using the ideal gas law.
Since the process is isothermal (constant temperature), we can use the relationship:
P1V1=P2V2
Where V2 is the volume of gas that flows out of the cylinder over time t.
Step 4: Express V2 in terms of flow rate and time.
Given the flow rate:
- Flow rate = 2.4L/min=2.4×10−3m3/min
Thus,
V2=Flow rate×t=(2.4×10−3)×t
Step 5: Substitute V2 into the ideal gas equation.
Substituting V2 into the equation:
P1V1=P2(2.4×10−3×t)
Step 6: Rearrange the equation to solve for t.
Rearranging gives:
t=P1V1P2×2.4×10−3
Step 7: Plug in the values and calculate t.
Substituting the known values:
t=(1.38×107)×(16×10−3)(105)×(2.4×10−3)
Calculating:
t=(1.38×107)×(16×10−3)(105)×(2.4×10−3)
t=(1.38×16)×1042.4
t=22.08×1042.4
t=9.2×103 minutes
t≈920 minutes
Step 8: Convert minutes to hours.
t≈92060≈15.33 hours
Conclusion
The cylinder will last for nearly 15 hours.
---
Given:
- Volume V1=16L=16×10−3m3
- Initial temperature T=300K
- Initial pressure P1=1.38×107Pa
- Final pressure P2=Patm=105Pa
Since the process is isothermal (constant temperature), we can use the relationship:
P1V1=P2V2
Where V2 is the volume of gas that flows out of the cylinder over time t.
Given the flow rate:
- Flow rate = 2.4L/min=2.4×10−3m3/min
V2=Flow rate×t=(2.4×10−3)×t
Substituting V2 into the equation:
P1V1=P2(2.4×10−3×t)
Rearranging gives:
t=P1V1P2×2.4×10−3
Substituting the known values:
t=(1.38×107)×(16×10−3)(105)×(2.4×10−3)
t=(1.38×107)×(16×10−3)(105)×(2.4×10−3)
t=(1.38×16)×1042.4
t=22.08×1042.4
t=9.2×103 minutes
t≈920 minutes
t≈92060≈15.33 hours
The cylinder will last for nearly 15 hours.
टॉपर्स ने हल किए ये सवाल
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Multiple Corrects
28 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Assertion-Reasoning
6 Videos
- KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASES AND FIRST LAW OF THERMODYNAMICSEXERCISE - Subjective
22 Videos
- KINETIC THEORY OF GASESBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - KINETIC THEORY OF GASESEXERCISE - Compression
2 Videos
- LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONBOOK - CENGAGE PHYSICS ENGLISHCHAPTER - LINEAR AND ANGULAR SIMPLE HARMONIC MOTIONEXERCISE - Single correct anwer type
14 Videos
समान प्रश्न
For an ideal gas
View SolutionStatement I: A quasi -static process is so called bacause it is a sudden and large change of the system.
Statement II: An adiabatic process is not quasi- static because it is a sudden and large change of the system.
View SolutionFive grams of helium having rms speed of molecules 1000m/s and 24g of oxygen having rms speed of 1000m/s are introduced into a thermally isolated vessel. Find the rms speeds of helium and oxygen individually when thermal equilibrium is attained. Neglect the heat capacity of vessel.
View SolutionOne mole of an ideal monatomic gas undergoes a linear process from A to B , in which is pressure P and its volume V change as shown in figure .
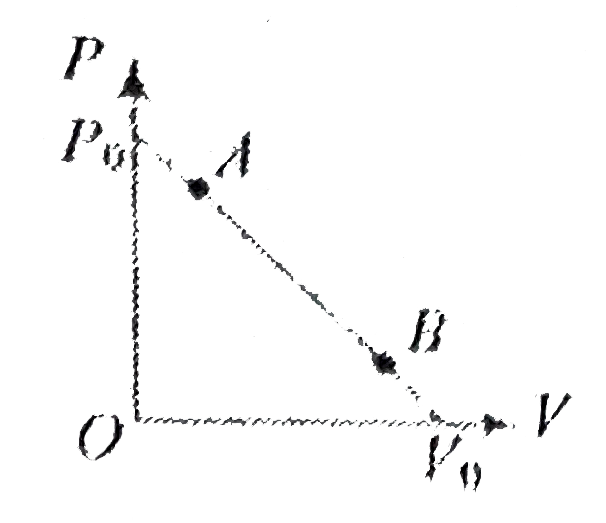
The absolute temperature T versus volume V for the given process is
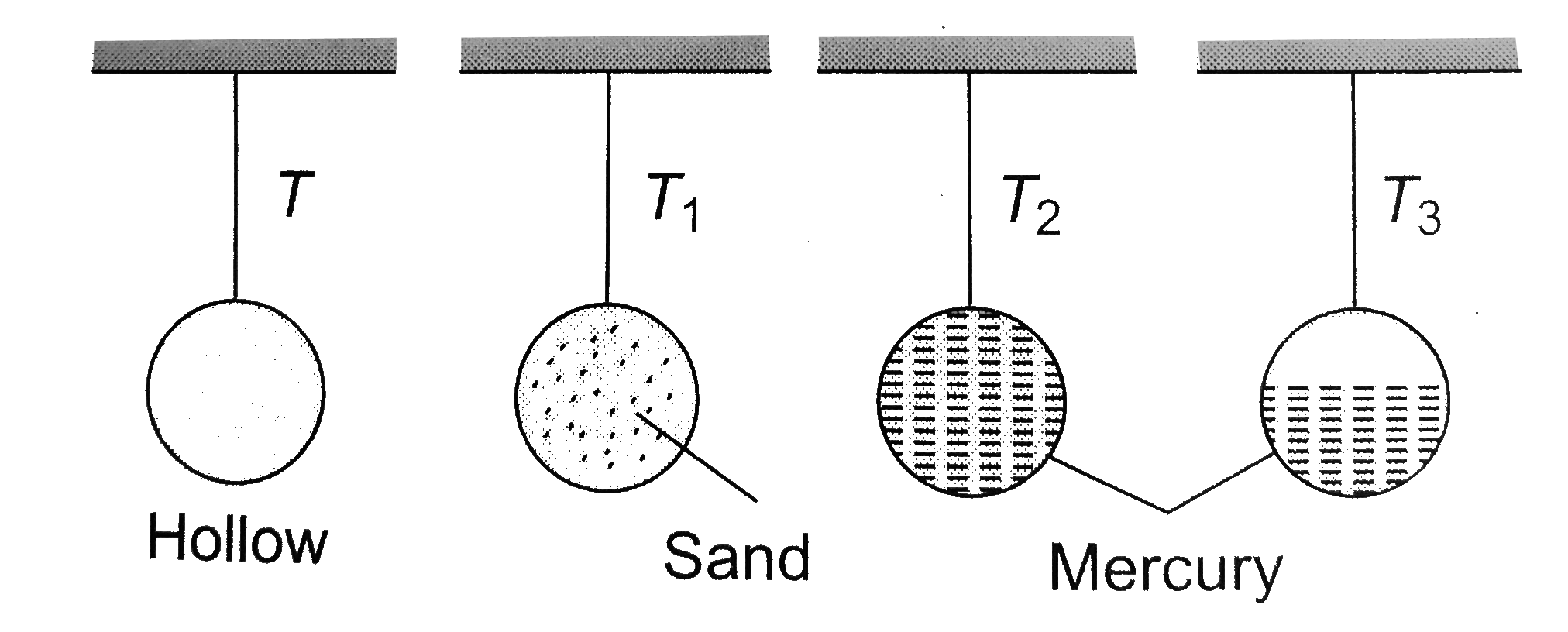
View SolutionThe period of a simple pendulum whose bob is hollow metallic sphere is T. The period is T1 when the bob is filled with sand, T2 where it is filled with mercury and T3 when it is half filled with mercury Which of the following is true?
View SolutionA stationary cylinder of oxygent used in a hospital has the following characteristics at room temperature 300K, gauge pressure 1.38×107 Pa. volume 16L. If the flow area, measured at atmospheric pressure, is constant at 2.4L/min, the cylinder will last for nearly
View SolutionAn iron cylider contains helium at a pressure of 250 kPa at 300 K The cylinder can withstand a pressure of 1 xx 10^(6) Pa The room in which cylinder is placed catches fire Predict whether the cylinder will blow up before it melts or not melting point of cylinder =1800 K .
View SolutionA cylinder of oxygen, used as medical aid, has a volume of 16 L. It is filled to a pressure of 1.37x×107Nm–2 above the atmospheric pressure at room temperature of 300 K. When in use, the flow rate at atmospheric pressure is 2.4L/min. How long will the cylinder last? Atmospheric pressure is 105Nm–2.
View SolutionAn iron cylinder contains helium at a pressure of 250 k Pa at 300 K . The cylinder can withstand a pressure of 1 xx 10^(5) Pa . The room in which the cylinder is placed catches fire. Predict whether the cylinder will blow before it melts or not (m.p of cylinder = 1800 K )
View SolutionA compressed cylinder of gas contains 1.50×103 g of N2 gas at a pressure of 2.0×107 Pa and a temperature of 17.1∘C. What volume of gas has been released into the atmosphere if the final pressure in the cylinder is 1.80×105 Pa ? Assume ideal behaviour and that the gas temperature is unchanged.
View Solutionलोहे के किसी सिलिण्डर में 300 K ताप तथा 230 kPa दाब पर He भरी है | यह सिलिण्डर 1×106Pa का दाब सहन कर सकता है | जिस कमरे में यह सिलिण्डर रखा है उसमे आग लग जाती है | यदि धातु का गलनांक 1800 K हो तो ज्ञात करो क्या यह सिलिण्डर गलने से पहले फट जायेगा ?
View SolutionA cylinder contains oxygen at pressure of 28 atm and at a temperature of 300 K. the mass of oxygen the gas, the pressure is found to be 7 atm and the temperature is 200 K. what mass of the gas has been used?
View SolutionA cylinder containing cooking gas can withstand a pressure of 15atm. The pressure gauge of the cylinder indicates 12atm at 27∘C. Due to a sudden fire in the building, the temperature starts rising. At what temperature will the cylinder explode?
View SolutionAn iron cylinder contains helium at a pressure of 250 k pa and27∘C. The cylinder can withstand a pressure of 1×106pa. The room in which cylinder is palced catches fire. Predict whether the cylinder will blow up before it melts or not. [melting point of cylinder =1800 k]
View SolutionA hydrogen cylinder can withstand an internal pressure of 7 times 10^6 Pa . The pressure of hydrogen inder at 15^@C is 1.7 times 10^6 Pa. At what minimum temperature an explosion may take place?
View Solution

