An organic compound (A) with molecular formula C7H8O dissolves in NaOH and gives characteristic colour with FeCl3. On treatement with Br3, it gives a tribromo product C7H5Br3. The compound is:
p-Hydroxybenzene
2-Methoxy-2-phenyl propane
m-Cresol
p-Cresol
The correct Answer is:C
To solve the question regarding the organic compound (A) with the molecular formula C₇H₈O, we will follow these steps:
Step 1: Determine the Degree of Unsaturation
The degree of unsaturation (DU) can be calculated using the formula:
DU=2C+2−H2
where C is the number of carbon atoms and H is the number of hydrogen atoms.
For the compound C₇H₈O:
- C = 7
- H = 8
Substituting the values:
DU=2(7)+2−82=14+2−82=82=4
Step 2: Analyze the Structure
A degree of unsaturation of 4 suggests the presence of a benzene ring (which accounts for 4 degrees of unsaturation). This indicates that the compound likely has a benzene ring and may also have additional substituents.
Step 3: Identify Functional Groups
The presence of an -OH group (hydroxyl group) is indicated by the molecular formula C₇H₈O. Given that the compound dissolves in NaOH and gives a characteristic color with FeCl₃, this suggests that the compound is a phenol.
Step 4: Consider Substituents on the Benzene Ring
Since the compound gives a tribromo product (C₇H₅Br₃) upon treatment with Br₂, this indicates that bromine is adding to the benzene ring. The formation of a tribromo product suggests that both ortho and para positions on the ring are available for substitution, which implies that a methyl group (–CH₃) is likely present at the meta position relative to the hydroxyl group.
Step 5: Identify the Compound
Considering the structure we have deduced:
- The compound is a phenol (due to the -OH group).
- The methyl group is at the meta position relative to the -OH group.
This compound is known as meta-cresol or metacresol.
Conclusion
The organic compound (A) is metacresol.
The degree of unsaturation (DU) can be calculated using the formula:
DU=2C+2−H2
where C is the number of carbon atoms and H is the number of hydrogen atoms.
- C = 7
- H = 8
DU=2(7)+2−82=14+2−82=82=4
A degree of unsaturation of 4 suggests the presence of a benzene ring (which accounts for 4 degrees of unsaturation). This indicates that the compound likely has a benzene ring and may also have additional substituents.
The presence of an -OH group (hydroxyl group) is indicated by the molecular formula C₇H₈O. Given that the compound dissolves in NaOH and gives a characteristic color with FeCl₃, this suggests that the compound is a phenol.
Since the compound gives a tribromo product (C₇H₅Br₃) upon treatment with Br₂, this indicates that bromine is adding to the benzene ring. The formation of a tribromo product suggests that both ortho and para positions on the ring are available for substitution, which implies that a methyl group (–CH₃) is likely present at the meta position relative to the hydroxyl group.
Considering the structure we have deduced:
- The compound is a phenol (due to the -OH group).
- The methyl group is at the meta position relative to the -OH group.
The organic compound (A) is metacresol.
Topper's Solved these Questions
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Exercises Assertion-Reasoning
Explore 3 Videos
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Archives Single Correct
Explore 14 Videos
- ALCOHOL,PHENOL AND ETHERSBook:CENGAGE CHEMISTRY ENGLISHChapter:ALCOHOL,PHENOL AND ETHERSExercise:Exercises Multiple Correct
Explore 17 Videos
- ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESBook:CENGAGE CHEMISTRY ENGLISHChapter:ALIPHATIC AND AROMATIC ALDEHYDES AND KETONESExercise:Archives Subjective
Explore 26 Videos
Similar Questions
An organic compound (A) with molecular formula C7H8O dissolves in NaOH and gives characteristic colour with FeCl3. On treatment with Br2, it gives a tribromo product C7H5OBr3. The compound is:
View SolutionCompound (A)C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2(A) forms compound (B)C7H5OBr3.
The most probable structure of compound A is :
View Solution
Knowledge Check
Compound (A) C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2 (A) forms compound (B) C7H5OBr3 .
The most probable structure of compound A is :A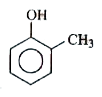 B
B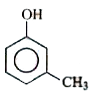 C
C D
D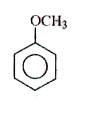
Compound (A) C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2 (A) forms compound (B) C7H5OBr3 .
The structure of compound (B) would be :A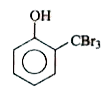 B
B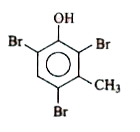 C
C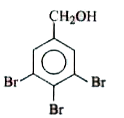 D
D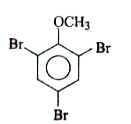
Compound (A) C7H8O is insoluble in aqueous NaHCO3 and dissolves in aqueous NaOH and gives a characteristic colour with neutral FeCl3 . When treated with Br2 (A) forms compound (B) C7H5OBr3 .
The most probable structure of compound A is :A B
B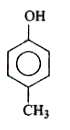 C
C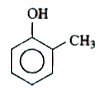 D
D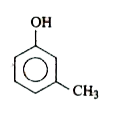
A compound (A) with molecular formula C5H10 gives one monochlorination product. Compound (A) is:
View SolutionThe molecular formula C7H8O represents the following except
View SolutionAn organic compound with molecular formula C6H12 upon ozonolysis gives only acetone as the product. The compound is :
View SolutionAn organic compound (X) has molecular formula C7H6O2
and it does not effervesces with NaHCO3. X on treatment with
excess of HBr(aq) gives Y(C6H6O2) and CH2Br2. Y forms a
violet coloured solution with FeCl3. If X treated with Br2Fe,
how many different monobromination product would result?
View SolutionAn organic compound 'A' having molecular formula C6H6O gives a characteristic colour with aqueous FeCl3 solution. When 'A' is treated with CO2 and NaOH at 400K under pressure. 'B' is obtained. B on acidification gives C when C treated with CH3COCl gives a popular pain killer D. Deduce the strucutrues of A,B,C and D.
View Solution
