An organic compound (X) has molecular formula C7H6O2
and it does not effervesces with NaHCO3. X on treatment with
excess of HBr(aq) gives Y(C6H6O2) and CH2Br2. Y forms a
violet coloured solution with FeCl3. If X treated with Br2Fe,
how many different monobromination product would result?
The correct Answer is:2
X is 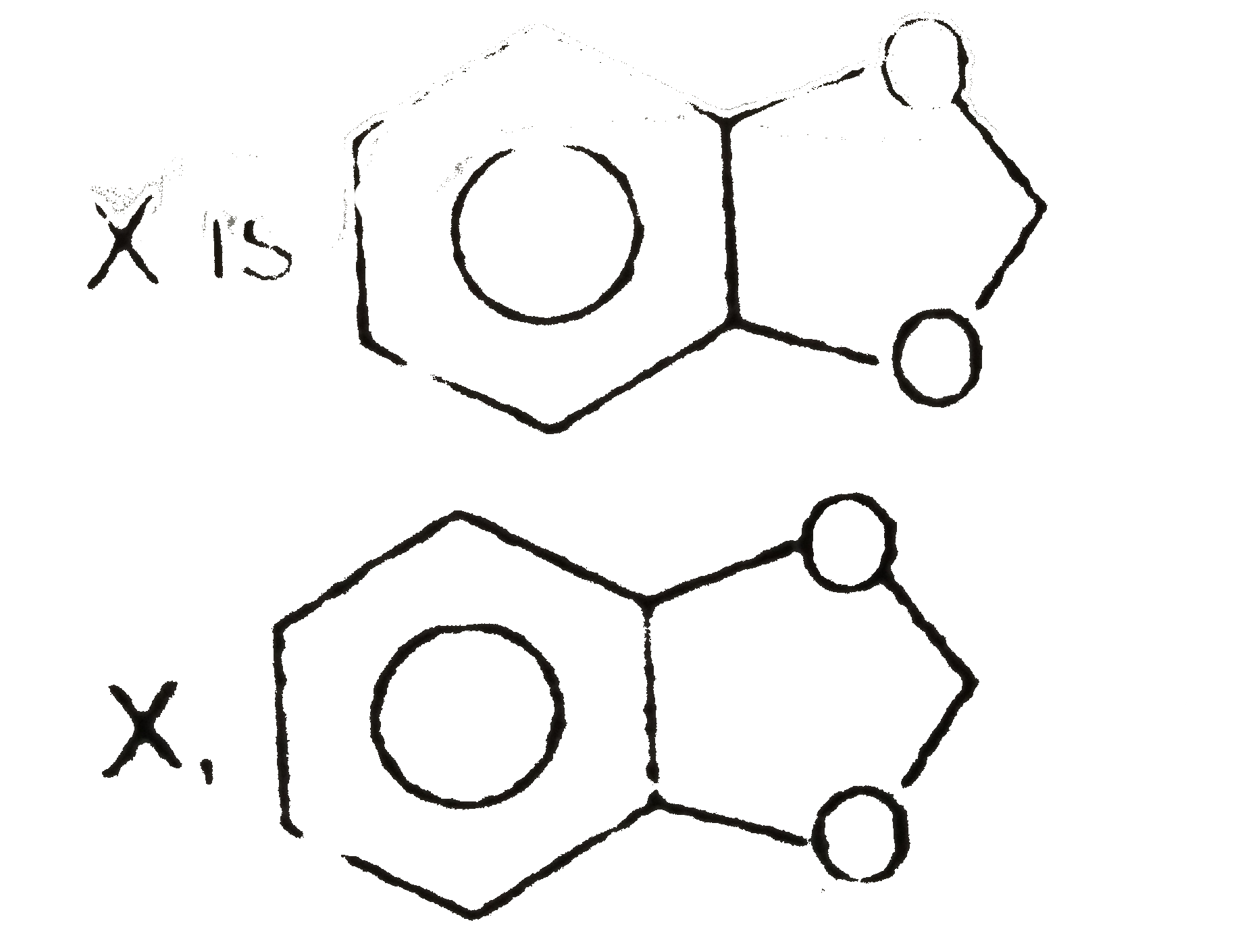
Topper's Solved these Questions
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:PART - III CHEMISTRY SEC - 1
Explore 12 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:PART - III CHEMISTRY SEC - 2
Explore 18 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
- TEST SERIESBook:RESONANCE ENGLISHChapter:TEST SERIESExercise:CHEMISTRY
Explore 50 Videos
Similar Questions
An organic compound (A) with molecular formula C7H8O dissolves in NaOH and gives characteristic colour with FeCl3. On treatment with Br2, it gives a tribromo product C7H5OBr3. The compound is:
View SolutionAn organic compound (A) with molecular formula C7H8O dissolves in NaOH and gives characteristic colour with FeCl3. On treatement with Br3, it gives a tribromo product C7H5Br3. The compound is:
View Solution
Knowledge Check
An optically active compound 'X' has molecular formula C4H8O3. If evolves CO2 withNaHCO3. 'X' reacts with LiAlH4 to give an achiral compound 'X' is -
ACH3CH2C∣OHHCOOHBCH3C∣MeHCOOHCCH3CHCOOH∣CH2OHDCH3C∣OHHCH2COOHAn organic compound A (Molecular formula C6H12O4) on treatment with Na metal liberates H2 gas and on treatment with HIO4 gives 2 moles of CH3CHO,HCOOH (1 mole) and CO2 (1 mole). Find out structure of A.
A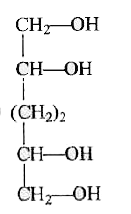 B
B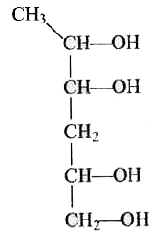 C
C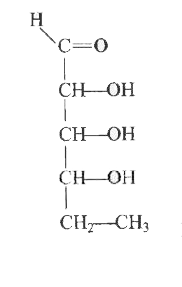 D
D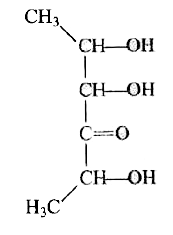
An unknown compound (A) has a molecular formula C4H6. When (A) is treated with excess of Br2 a new substance (B) with formula C4H6Br4 is formed. (A) forma a white ppt. with ammonical silver nitrate solution. (A) may be :
ABut-1-yneBBut-2-yneCBut-1-eneDBut-2-ene
An optically active compound 'X' has molecular formula C4H8O3. It evolves CO2 with NaHCO3. 'X' reacts with LiAIH4 to give an achiral compound 'X' is:
View Solution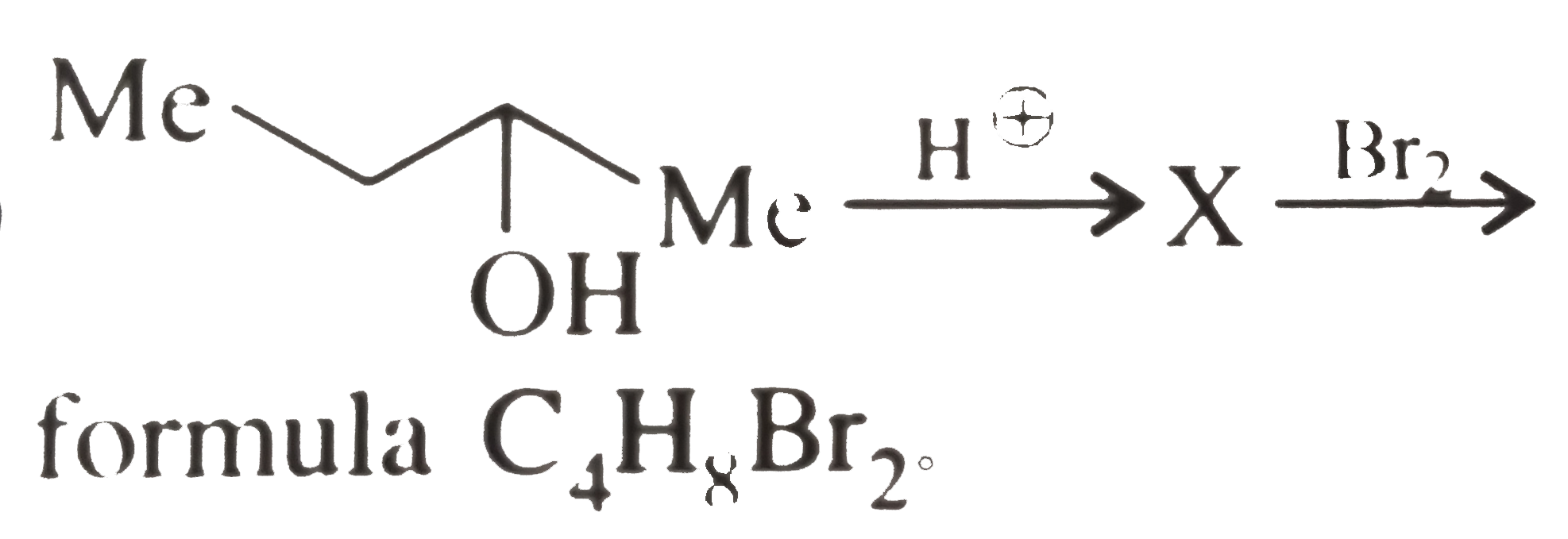
Find compound with formula C4H8Br2
How many structure of (X) are possible?
View Solution[Fe(CN)6]4− and [Fe(H2O)6]2+ are of different colours in dilute solutions why?
View Solution[Fe(CN)6]4− and [Fe(H2O)6]2+ are of different colours in dilute solutions why?
View SolutionAn organic compound 'X' with molecular formula C7H8O in insoluble in aqueous NaHCO3 but dissolved in NaOH. When treated with bromine water 'X' rapidly give 'Y' (C7H5OBr). The compound 'X' and 'Y' respectively are
View Solution
