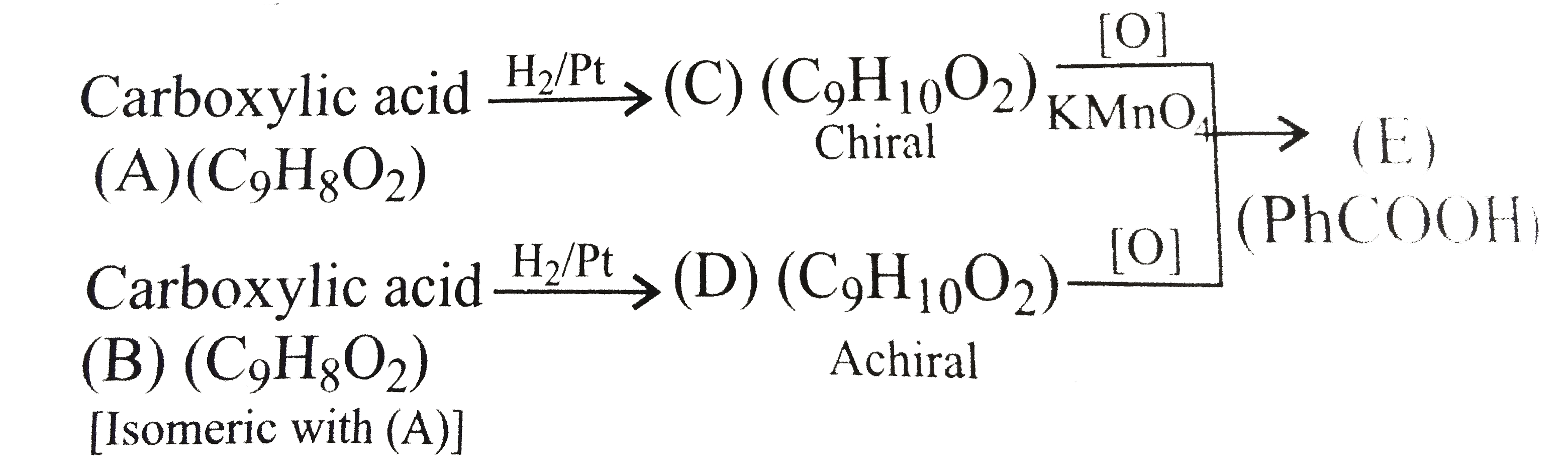
Compounds (C) and (D) can be distinguished by :
A
NaHCL3
B
H.V.Z. reaction
C
KMnO4/H⊕
D
AgNO3
ভিডিও সমাধান
লিখিত জবাব
The correct Answer is:B
(b) Formation of (E) suggests monosubstituted acid and reaction of 1mol of H2 with (A) and (B) shows one (C=C) bond. There are 6D.U.[4D.U.for(Ph) group, 1D.U. For (COOH), and 1D.U for (C=C)] in both (A) and (B).
Chiral compound with nine C atoms would be 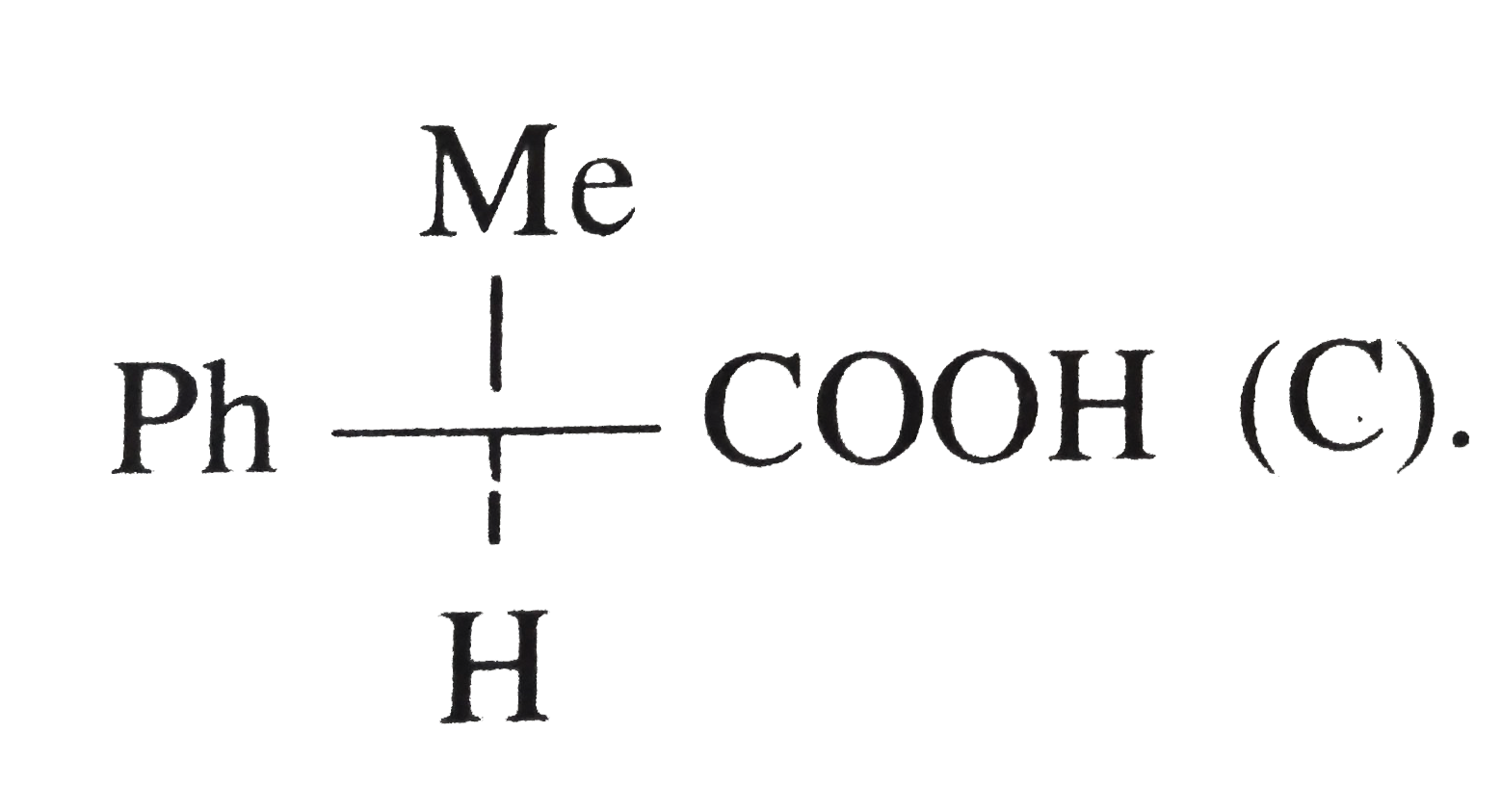
Reactions :
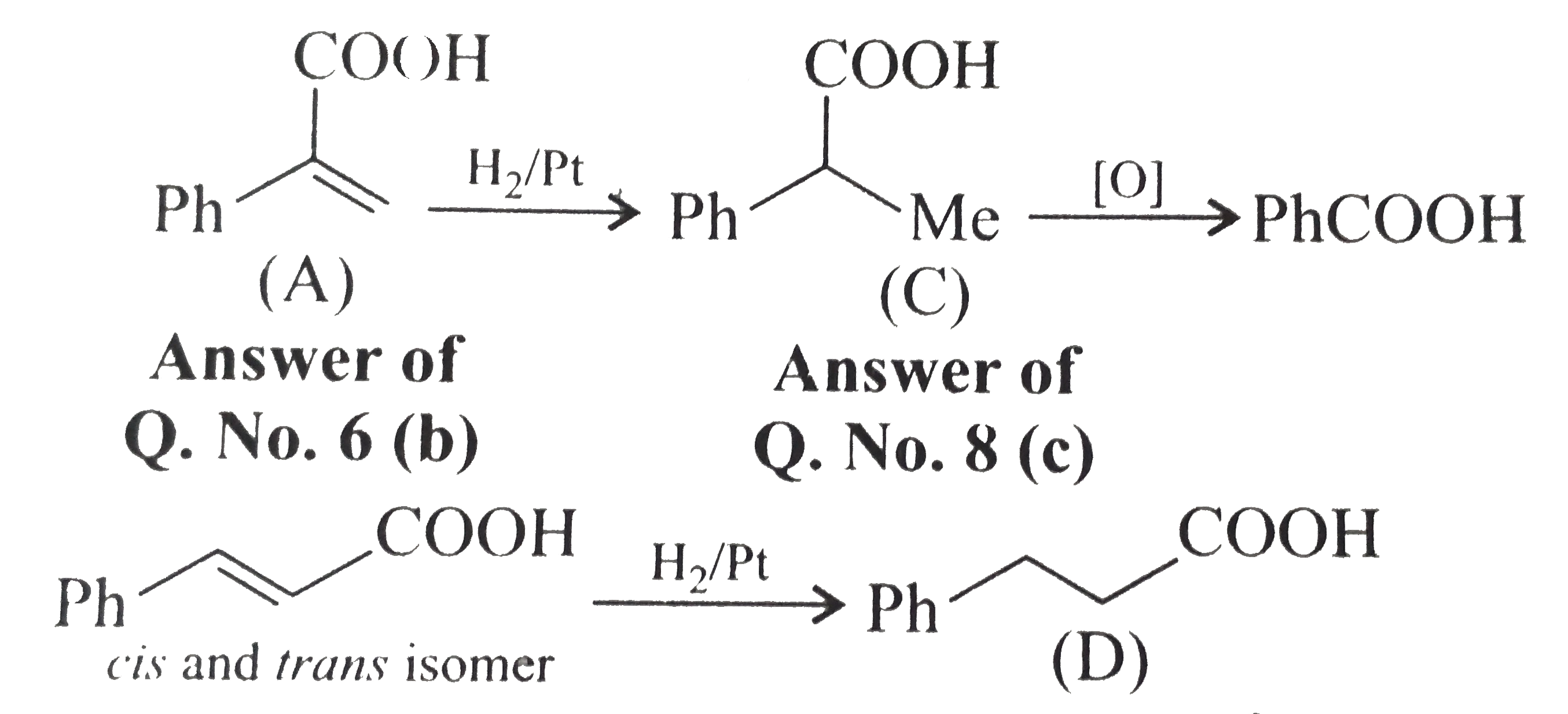
Here, (C) and (D) can be distinguised quantitatively by H.V.Z reaction (C) has one α−H atom and would give monochlorinaed product, whereas (D) has two α−H atoms and would give dichlorinated product. The chlorine content in the chlorinated derivatives of (C) and (D) is determined by quantative methods.
|
টপার এই প্রশ্নগুলো সমাধান করেছে
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:CENGAGE CHEMISTRY ENGLISHChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Exercises (Multiple Correct)
Explore 33 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:CENGAGE CHEMISTRY ENGLISHChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Exercises (Singlecorrect)
Explore 60 Videos
- CARBOXYLIC ACIDS AND THEIR DERIVATIVESBook:CENGAGE CHEMISTRY ENGLISHChapter:CARBOXYLIC ACIDS AND THEIR DERIVATIVESExercise:Exercises (Subjective)
Explore 19 Videos
- BIOMOLECULESBook:CENGAGE CHEMISTRY ENGLISHChapter:BIOMOLECULESExercise:Exercises Archives (Analytical And Descriptive)
Explore 5 Videos
- CHEMICAL KINETICSBook:CENGAGE CHEMISTRY ENGLISHChapter:CHEMICAL KINETICSExercise:Archives Subjective
Explore 22 Videos
সম্পর্কিত প্রশ্ন
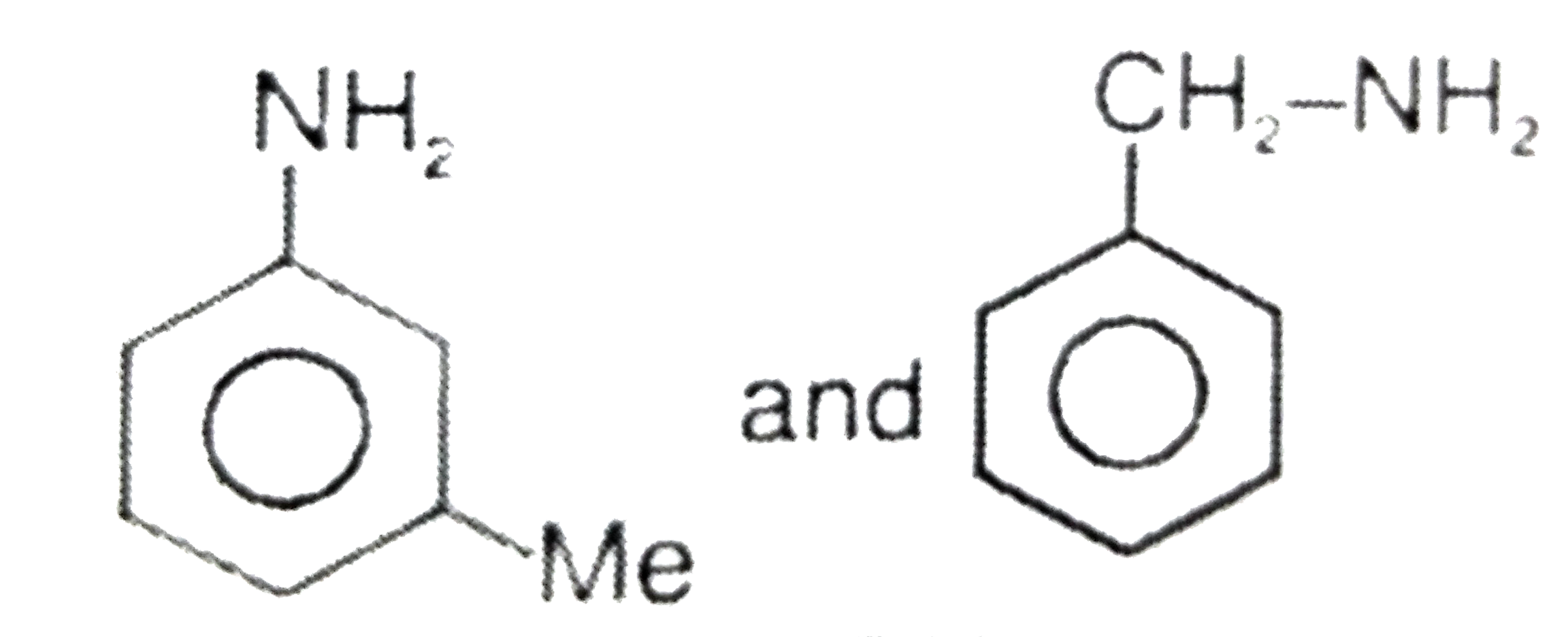 can be distinguish by
can be distinguish by
View Solution
Compound (X) and (Y) can be distinguished by
View SolutionH2SandSO2 can be distinguished by
View SolutionProduct(A) and (B)can be distinguished by
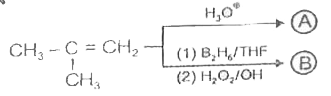
View Solution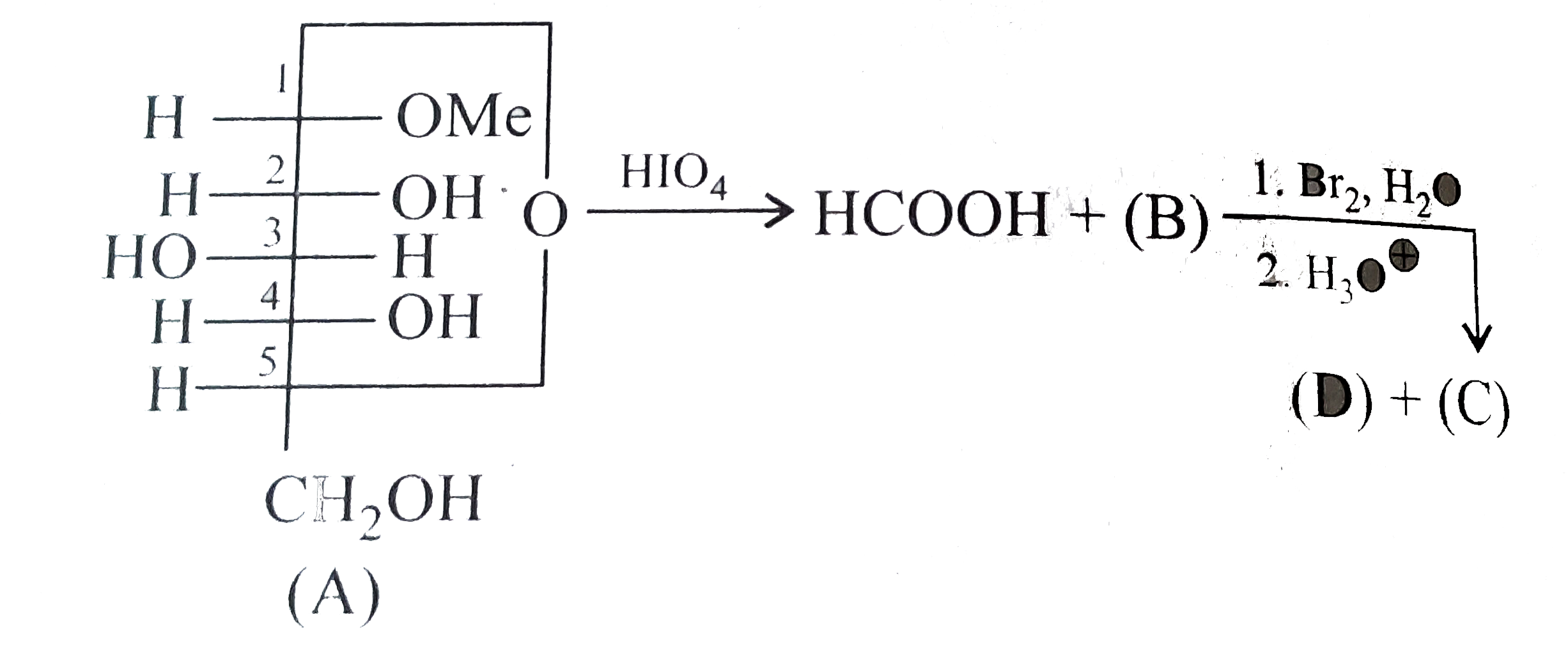
Compound (C) and (D) are:
View Solution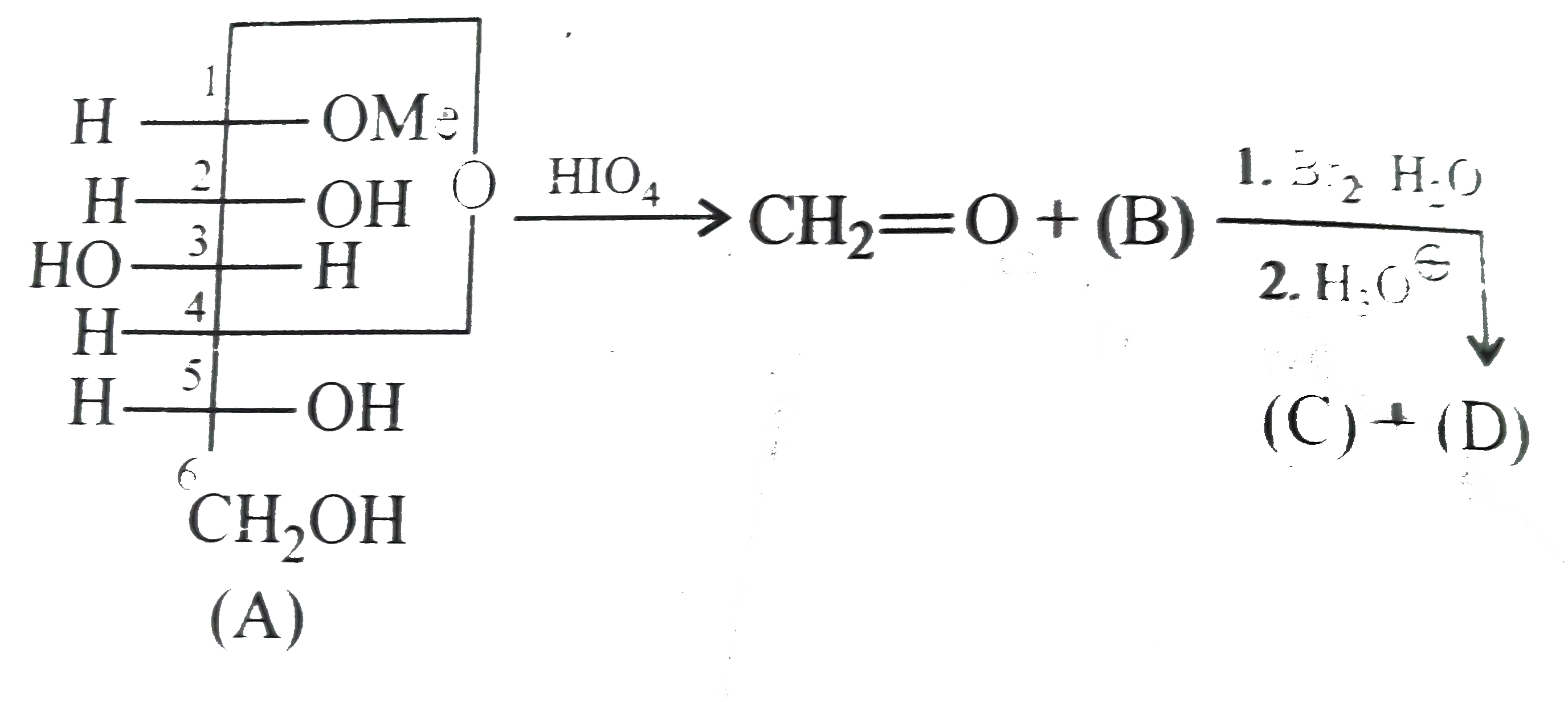
Compound (C) and (D) are:
View SolutionFe2+andFe3+ can be distinguished by
View SolutionThe following two compounds I and II can be distinguished by using reagent
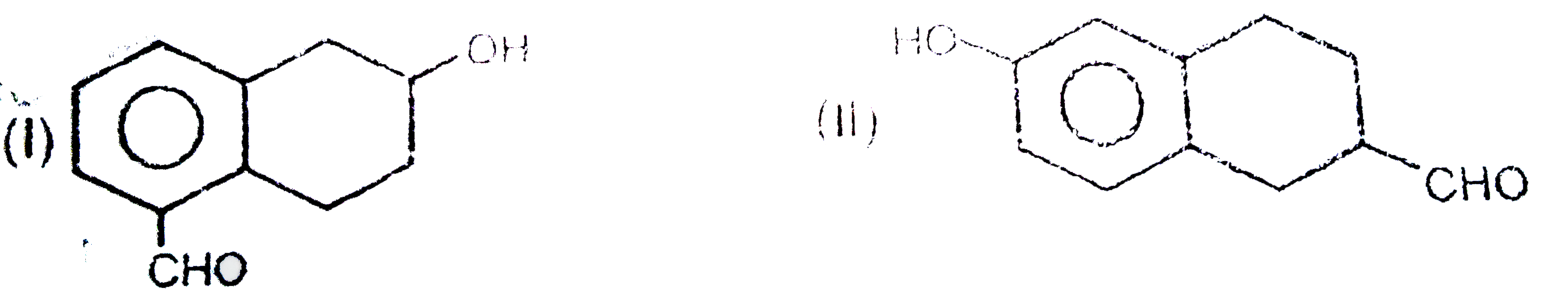
View Solution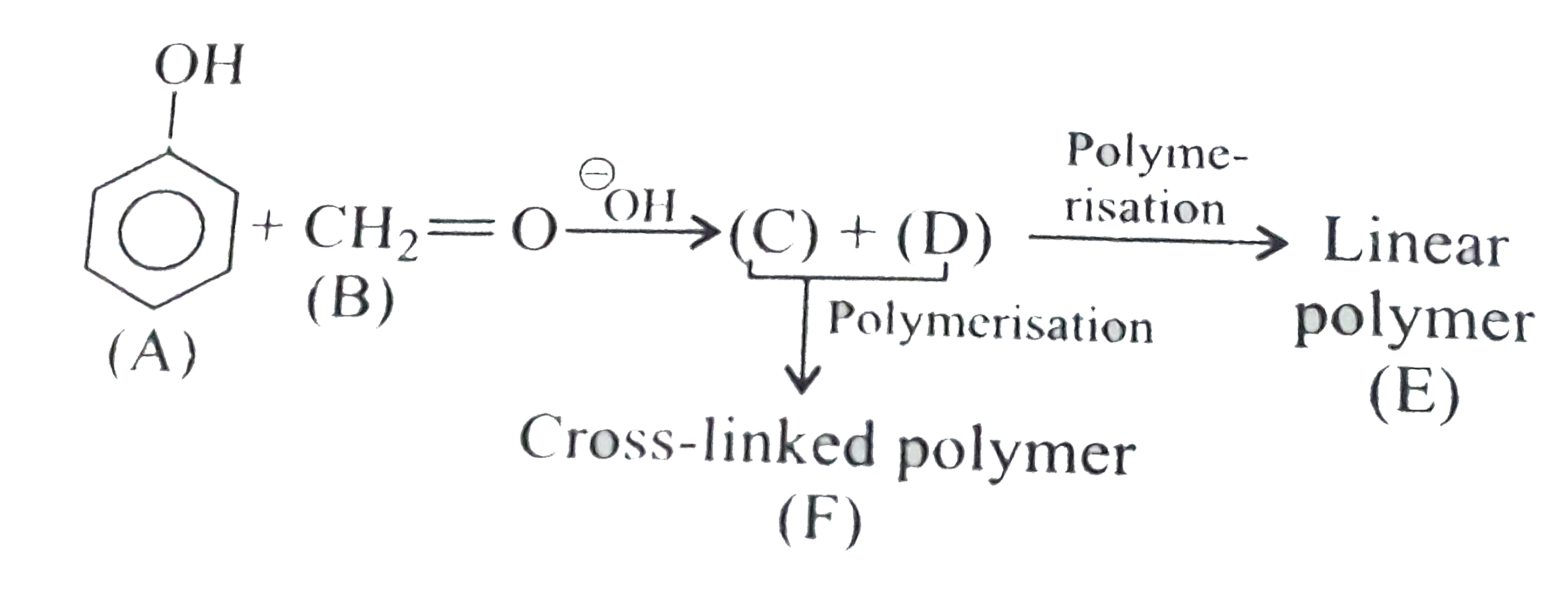
Compound (C ) and(D) are:
View SolutionThe following two compounds I and II can be distinguished by using reagent
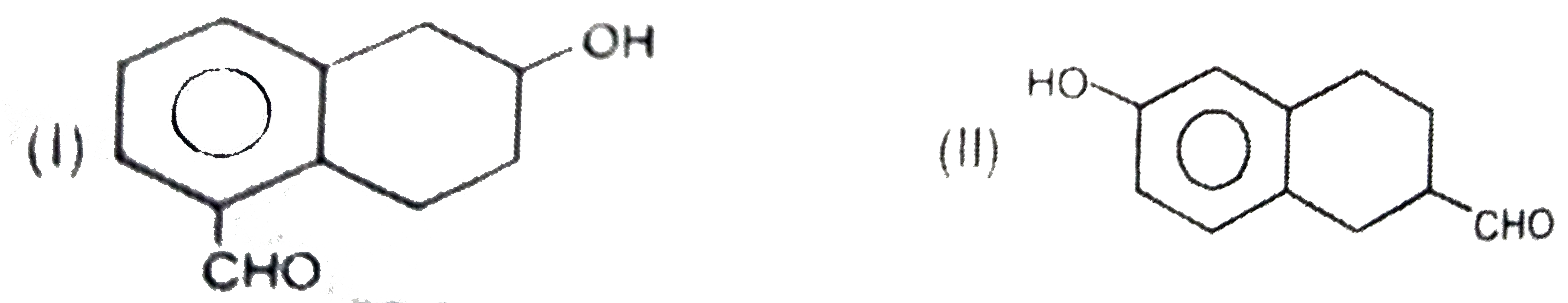
(a) aq. NaHCO3 , Neutral FeCl3
(b) Blue litmus solution , Na metal
(c) HCl/ZnCl2 anhydrous
View Solution
