A P−V graph for an ideal 'gas undergoing polytropic process PYm= Constant is sbown below. Find the value of m.
'(##CEN_KSR_PHY_JEE_C13_E01_015_Q05##)'
The correct Answer is:5
Topper's Solved these Questions
Similar Questions
Consider the four circuited shown in the figure, each consisting of a battry, a switch, a light bulb a resistor, and eutger a capacitory or an iductor. Assume the capacitor has a large capacitorand the inductor has a large inductance but no resitance. The light bulb has high efficiency. glowing whenever it carries electric current. (i) Describe what the light bulb does in each of each of curcuits (a) through (d) after the switch is thrown closed. (ii) Describe what the light bulb does in each of circuits (a) through (d) when, having been closed for a long time interval, the switch is opened.
View SolutionWhich of the following statements is correct in the given figure?
Infinitely long wire at E kept perpendicular to paper carrying current inwards:

View SolutionStraight line on (p - T) graph for an ideal gas represents isochoric process.
If p∝T,V=constant.
View SolutionP-V graph for an ideal gas undergoing polytropic process PV^(m) = constant is shown here. Find the value of m.
View SolutionFind the molar heat capacity of an ideak gas in a polytropic process pVn=const if the adiabatic exponent of the gas is equal to γ. At what values of the polytropic constant n will the heat capacity of the gas be negative ?
View Solution1 mol of an ideal gas undergoes different thermodynamical process in P-V diragram given below : What will be the value of DeltaH and DeltaE for overall process ?
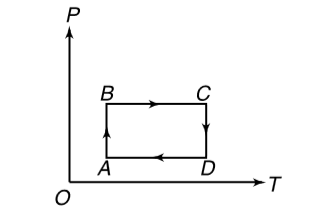
View SolutionAn ideal gas undergoes a cyclic process A→B→C→D for which P – T graph is as shown. Draw P – V and V – T of graph to represent the same process.
View SolutionFor an ideal gas the ratio of specific heats is (C_(p))/(C_(v)) = gamma . The gas undergoes a polytropic process PV^(n) = a constant. Find the values of n for which the temperature of the gas increases when it rejects heat to the surrounding.
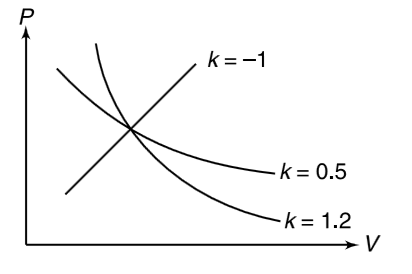
View SolutionFigure shows P versus V graph for various processes performed by an ideal gas. All the processes are polytropic following the process equation PVk= constant.
(i) Find the value of k for which the molar specific heat of the gas for the process is CP+CV2. Does any of the graph given in figure represent this process?
(ii) Find the value of k for which the molar specific heat of the gas is CV+CP. Assume that gas is mono atomic. Draw approximately the P versus V graph for this process in the graph given above.
View SolutionAn ideal gas with heat capacity at constant volume C_(V) undergoes a quasistatic process described by P V^(alpha) in a P-V diagram, where alpha is a constant. The heat capacity of the gas during this process is given by-
View SolutionFor polytropic process PVn = constant, molar heat capacity (Cm) of an ideal gas is given by:
View SolutionIdeal gas and polytropic process
View SolutionFor polytropic process PVn = constant, Cm (molar heat capacity) of an ideal gas is given by
View SolutionFor polytropic process PVn = constant, Cm (molar heat capacity) of an ideal gas is given by
View SolutionStraight line on (p - T) graph for an ideal gas represents isochoric process. If p prop T, V = constant .
View Solution

