STATEMENT-1: In the reaction : Cl2+OH−→Cl−+ClO−4 chlorine is oxidised only
STATEMENT-2: Oxidation and reduction cannot take place alone.
The correct Answer is:4
To analyze the statements provided in the question, we will break down the reaction and evaluate the oxidation states of chlorine in each species involved.
1. Identify the Reaction:
The reaction given is:
Cl2+OH−→Cl−+ClO−4
2. Determine Oxidation States:
- In Cl2, the oxidation state of chlorine (Cl) is 0 (elemental form).
- In Cl−, the oxidation state of Cl is -1.
- In ClO−4, we need to calculate the oxidation state of Cl. The overall charge is -1, and oxygen has an oxidation state of -2. Since there are four oxygens:
Let the oxidation state of Cl be x.
x+4(−2)=−1⟹x−8=−1⟹x=+7.
Thus, the oxidation states are:
- Cl in Cl2: 0
- Cl in Cl−: -1
- Cl in ClO−4: +7
3. Identify Oxidation and Reduction:
- Oxidation is defined as an increase in oxidation state. Here, Cl changes from 0 in Cl2 to +7 in ClO−4, indicating oxidation.
- Reduction is defined as a decrease in oxidation state. Here, Cl changes from 0 in Cl2 to -1 in Cl−, indicating reduction.
4. Evaluate Statement 1:
- The statement claims that "chlorine is oxidized only." However, since chlorine is both oxidized (to +7) and reduced (to -1) in this reaction, Statement 1 is incorrect.
5. Evaluate Statement 2:
- The statement claims that "oxidation and reduction cannot take place alone." This is not true, as there are reactions where only oxidation or only reduction occurs. For example:
- Oxidation: Na→Na++e− (only oxidation).
- Reduction: Cl2+2e−→2Cl− (only reduction).
Therefore, Statement 2 is also incorrect.
Conclusion:
Both statements are incorrect.
The reaction given is:
Cl2+OH−→Cl−+ClO−4
- In Cl2, the oxidation state of chlorine (Cl) is 0 (elemental form).
- In Cl−, the oxidation state of Cl is -1.
- In ClO−4, we need to calculate the oxidation state of Cl. The overall charge is -1, and oxygen has an oxidation state of -2. Since there are four oxygens:
Let the oxidation state of Cl be x.
x+4(−2)=−1⟹x−8=−1⟹x=+7.
Thus, the oxidation states are:
- Cl in Cl2: 0
- Cl in Cl−: -1
- Cl in ClO−4: +7
- Oxidation is defined as an increase in oxidation state. Here, Cl changes from 0 in Cl2 to +7 in ClO−4, indicating oxidation.
- Reduction is defined as a decrease in oxidation state. Here, Cl changes from 0 in Cl2 to -1 in Cl−, indicating reduction.
- The statement claims that "chlorine is oxidized only." However, since chlorine is both oxidized (to +7) and reduced (to -1) in this reaction, Statement 1 is incorrect.
- The statement claims that "oxidation and reduction cannot take place alone." This is not true, as there are reactions where only oxidation or only reduction occurs. For example:
- Oxidation: Na→Na++e− (only oxidation).
- Reduction: Cl2+2e−→2Cl− (only reduction).
Therefore, Statement 2 is also incorrect.
Both statements are incorrect.
Topper's Solved these Questions
- REDOX REACTIONSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - REDOX REACTIONSEXERCISE - Assignment (Section F) (Matrix-Match Type Questions)
4 Videos
- REDOX REACTIONSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - REDOX REACTIONSEXERCISE - Assignment (Section G) (Interger Answer Type Questions)
8 Videos
- REDOX REACTIONSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - REDOX REACTIONSEXERCISE - Assignment (Section D) (Linked Comprehension Type Questions)
12 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - PRINCIPLES OF QUALITATIVE ANALYSISEXERCISE - Assignment (SECTION H)
9 Videos
- SOLUTIONSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - SOLUTIONSEXERCISE - ASSIGMENT (SECTION-J) AAKASH CHALLENGERS QUESTIONS
10 Videos
Similar Questions
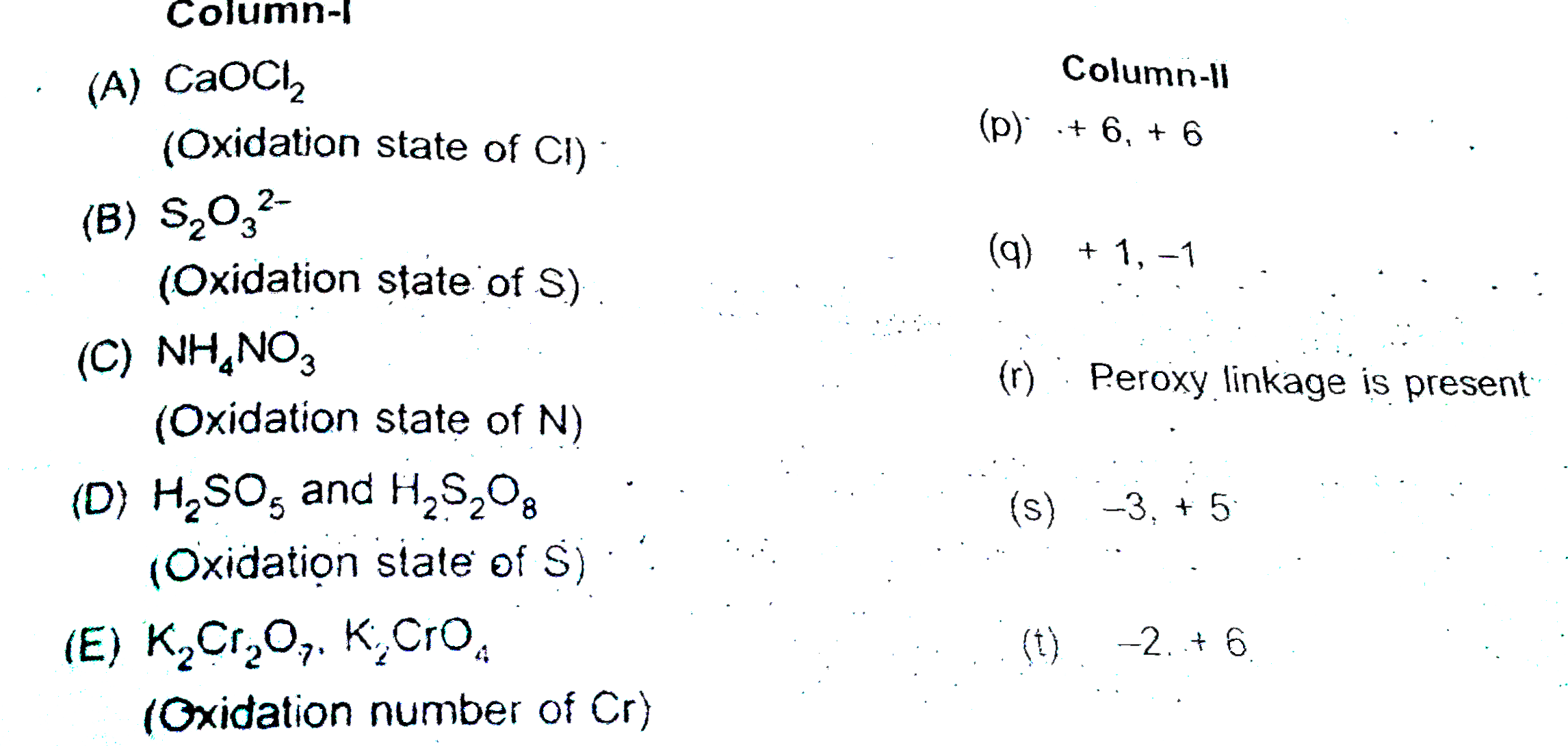
Match Column-I with Column-II
View Solution0.144 g of pure FeC2O4 was dissolvedin dilute H2SO4 and the solution was diluted to 100 ml . What volume in ml of 0.1 M KMnO4 will be needed to oxidise FeC2O4 solution
View SolutionRedox is a reaction in which both oxidation and reduction will take place simultaneously . It is obvious that if one substance gives electron there must be another substance to accept these electrons . In some reactions, same substance is reduced as well as oxidised, these reactions are termed as disproportionation reactions.
For calculating equivalent mass in redox reaction change in oxidtaion number is realted to n-factor which is reciprocal of molar ratio.
This reaction is an example of
Br2+OH−→BrO−3+H2O+Br−
View SolutionWhen a white powder (A) is strongly heated, it gives of a colourless, odourless gas (B) which turns lime water milky (C) and if the passage of this gas is continued the milkiness disappears and gives a solution (D). The solid residue (E) is yellow when hot, but turns white on cooling. Idnetify (A) to (E) with help of the equations.
View SolutionAn aqueous solution of H2SO4 has density 1.84 g/ml. Solution contains 98% H2SO4 by mass.Calculate
(i) Molarity of solution
(ii) Molar volume of solution
(iii) Relative lowering of vapour pressure w.r.t. water, assuming H2SO4 as non-electrolyte at this high concentration.
View SolutionIn the reaction, Cl_(2)+OH^(-)rarrCl^(-)+ClO_(4)^(-1)+H_(2)O , chlorine is:
View SolutionStatement-I: Absolute value of E0red of an electrode cannot be determined.
Because Statement-II: Neither oxidation nor reduction can take place alone.
View SolutionIn the reaction :Cl_(2)+OH^(-)rarrCl^(-)+ClO_(4)^(-)+H_(2)O :-
View SolutionStatement-1: Absolute value of E_("red")^(@) of an electrode cannot be determined. statement-2: Neither oxidation nor reduction can take place alone.
View SolutionOxidation and reduction process involves the transaction of electrons. Loss of electrons is oxidation and the gain of electrons is reduction. It is thus obvious that in a redox reaction, the oxidant is reduced by accepting the electrons and the reductant is oxidised by losing electrons. The reactions in which a species disproportionates into two oxidation states (lower and higher) are called disproportionation reactions. In electrochemical cells, redox reaction is involved, i.e., oxidation takes place at anode and reduction at cathode.
The reaction Cl2→Cl−+ClO−3 is:
View SolutionStatement-I:


Statement-II Phenol cannot be chlorinated because the ring is susceptible to oxidation by Cl2.
View SolutionStatement-2: Absolute value of E0red of an electrode cannote be determined.
Statement-2: Neither oxidation nor reduction can take place alone.
View SolutionSTATEMENT-1: In the reaction : Cl_(2)+ OH^(-) to Cl^(-) + ClO_(4)^(-) chlorine is oxidised only STATEMENT-2: Oxidation and reduction cannot take place alone.
View SolutionCl_(2) disproportionate into Cl^(-) and ClO_(3) in alkali.The equivalent weight of cl_(2) in this reaction is
View SolutionBalance the following reactions by ion electron method : Cl_(2) + OH^(-) rarr ClO_(3)+Cl + H_(2)O
View Solution

