Which defect causes decreases in the density of crystal?
a)Frenkel
b)Schottky
c)Interstitial
d)F-centre
The correct Answer is:B
To solve the question of which defect causes a decrease in the density of a crystal, we can follow these steps:
Step 1: Understand the Concept of Crystal Defects
- Crystals can have defects that affect their properties, including density. The main types of defects include vacancies, interstitials, and substitutional defects.
Hint: Familiarize yourself with the different types of crystal defects and their characteristics.
Step 2: Identify the Types of Defects
- The common types of defects are:
1. Vacancy Defect: Missing ions in the crystal lattice.
2. Interstitial Defect: Extra ions occupying spaces between the regular lattice positions.
3. Substitutional Defect: Atoms in the lattice are replaced by different atoms.
4. Schottky Defect: A type of vacancy defect where an equal number of cations and anions are missing.
Hint: Recall the definitions and implications of each defect type.
Step 3: Analyze the Schottky Defect
- The Schottky defect involves the creation of vacancies for both cations and anions. This means that there are fewer ions in the unit cell.
Hint: Consider how the removal of ions (vacancies) affects the overall mass of the unit cell.
Step 4: Relate Mass, Volume, and Density
- Density is defined as mass per unit volume (Density = Mass/Volume). If the number of ions (and thus the mass) decreases while the volume remains constant, the density will also decrease.
Hint: Remember the formula for density and how changes in mass affect it.
Step 5: Conclusion
- Since the Schottky defect leads to a decrease in the number of ions in the unit cell, it results in a decrease in mass while the volume remains unchanged. Therefore, the density of the crystal decreases.
Final Answer: The defect that causes a decrease in the density of the crystal is the Schottky defect.
- Crystals can have defects that affect their properties, including density. The main types of defects include vacancies, interstitials, and substitutional defects.
- The common types of defects are:
1. Vacancy Defect: Missing ions in the crystal lattice.
2. Interstitial Defect: Extra ions occupying spaces between the regular lattice positions.
3. Substitutional Defect: Atoms in the lattice are replaced by different atoms.
4. Schottky Defect: A type of vacancy defect where an equal number of cations and anions are missing.
- The Schottky defect involves the creation of vacancies for both cations and anions. This means that there are fewer ions in the unit cell.
- Density is defined as mass per unit volume (Density = Mass/Volume). If the number of ions (and thus the mass) decreases while the volume remains constant, the density will also decrease.
- Since the Schottky defect leads to a decrease in the number of ions in the unit cell, it results in a decrease in mass while the volume remains unchanged. Therefore, the density of the crystal decreases.
Topper's Solved these Questions
- THE SOLID STATE BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THE SOLID STATE EXERCISE - ASSIGNMENT SECTION-B (OBJECTIVE)
27 Videos
- THE SOLID STATE BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THE SOLID STATE EXERCISE - ASSIGNMENT SECTION-C (OBJECTIVE)
17 Videos
- THE SOLID STATE BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THE SOLID STATE EXERCISE - Try Yourself
56 Videos
- THE S-BLOCK ELEMENTSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THE S-BLOCK ELEMENTSEXERCISE - Assignment (Section-J)
10 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - ASSIGNMENT (Section -D) Assertion-Reason Type Questions
15 Videos
Similar Questions
Why does Schottky defect decrease the desity of a crystal ?
View Solutionwhich of the following statement are not true ?
(a) Vacancy defect results in a decrease in the density of the substance
(b)interstitial defects results in an increase in the density of the substance
(c)impurity defect has no effect on the density of the substance
(d)Frenkel defect results results in an increase in the density of the substance
View SolutionIonic lattic has two major points defects ,(1) Schottky (2) Frenkel defects . Schottkly defects occurs due to the cations - anion pair's missing from the lattice sites . Frenkel defects occurs when cation leaves its lattice site and fits into an interstitial space. The neutrality of the crystal is being maintained and we considered all losses from interstitial positions.
which defect decrease density of the crystal ?
View SolutionWhich stoichiometric defect in crystals increses the density a solid?
View SolutionWhich point defect lowers the density of a crystal?
View SolutionWhat is the effect of Schottky defect on the density of crystal ?
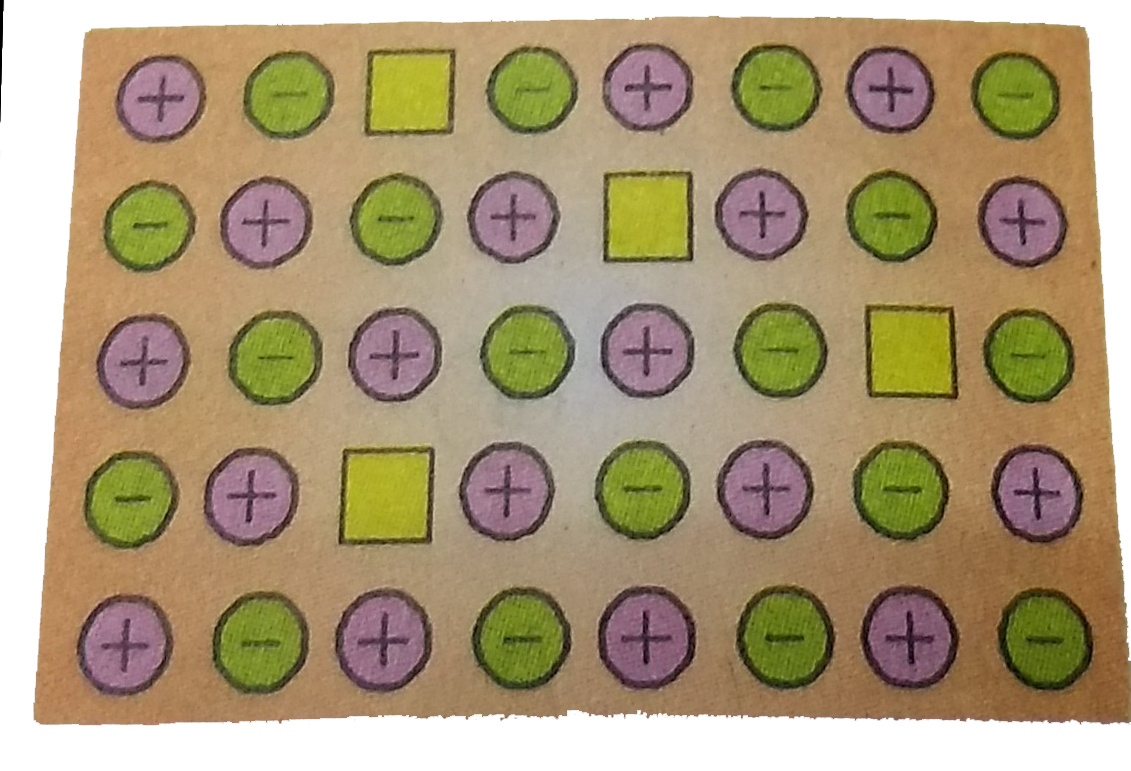
View SolutionExamine the illustration of a portion of the deffective crystal and answer the following question :
(a) What are these type of vacancy defects called ?
(b) How is the density of a crystal affected by these defects ?
(c) Name one ionic compound which can show this type of defect in the crystalline state.
(d) How is the stoichiometry of the compound affected ?
View SolutionWhich of the following defects, if present, lowers the density of the crystal ?
View SolutionExamine the defective crystal given below and answer the question that follows :

State if the above defect is stoichiometric or non-stoichiometric. How does this defect affect the density of the crystal ? Also, write the term used for this type of defect.
View SolutionDefects which do not disturb the stoichiometry are called stochiometric defect. At the time of crystal formation an ion may not occupy its expected position leaving a void or hole at that site. As a metter of fact one positive and one negative ion are missing from their respective positions leaving behind a pair of holes. This vacancy is a Schottky defect. Due to this defect density decreases. Defect arises in which certain atoms or ions have migrated to interstitial position some distance removed from the holes which they vacated. In this case there is no change in the density of the crystal.
Frenkel defect is generally observed in
View Solution

