What is the effect of Schottky defect on the density of crystal ?
Step-by-Step Solution:
1. Understanding Schottky Defect:
- A Schottky defect occurs in a crystal when equal numbers of cations and anions are missing from their lattice positions. This creates vacancies in the crystal structure.
2. Example of Schottky Defect:
- Consider the sodium chloride (NaCl) crystal structure. In this structure, if one Na⁺ ion and one Cl⁻ ion are removed, it results in a vacancy. This is a classic example of a Schottky defect.
3. Effect on Mass:
- When cations and anions are removed from the crystal lattice, the overall mass of the crystal decreases because some of the constituent ions are missing.
4. Effect on Volume:
- The volume of the crystal remains the same since the crystal structure is still intact, despite the missing ions.
5. Calculating Density:
- Density is defined as mass per unit volume (Density = Mass/Volume). Since the mass of the crystal decreases while the volume remains unchanged, the density of the crystal will decrease.
6. Conclusion:
- Therefore, the presence of Schottky defects in a crystal leads to a decrease in its density.
- A Schottky defect occurs in a crystal when equal numbers of cations and anions are missing from their lattice positions. This creates vacancies in the crystal structure.
- Consider the sodium chloride (NaCl) crystal structure. In this structure, if one Na⁺ ion and one Cl⁻ ion are removed, it results in a vacancy. This is a classic example of a Schottky defect.
- When cations and anions are removed from the crystal lattice, the overall mass of the crystal decreases because some of the constituent ions are missing.
- The volume of the crystal remains the same since the crystal structure is still intact, despite the missing ions.
- Density is defined as mass per unit volume (Density = Mass/Volume). Since the mass of the crystal decreases while the volume remains unchanged, the density of the crystal will decrease.
- Therefore, the presence of Schottky defects in a crystal leads to a decrease in its density.
Similar Questions
Knowledge Check
What is the effect of Frenkel defect on the density of ionic solids?
AThe density of the crystal increasesBThe density of the crystal decreasesCThe density of the crystal remains unchangedDThere is no relationship between density of a crystal and defect present in itIn the Schottky defect:
Aan ion moves to interstitial position between the lattice pointsBelectrons are trapped in a lattice siteCsome lattice sites are vacantDsome extra cations are present in interstitial spaces
Which point defect lowers the density of a crystal?
View SolutionFrenkel defect does not change the density of the ionic crystal whereas, Schottky defect lowers the density of ionic crystal. Give a reason.
View SolutionWhich defect causes decreases in the density of crystal?
View SolutionWhich defect causes decreases in the density of crystal?
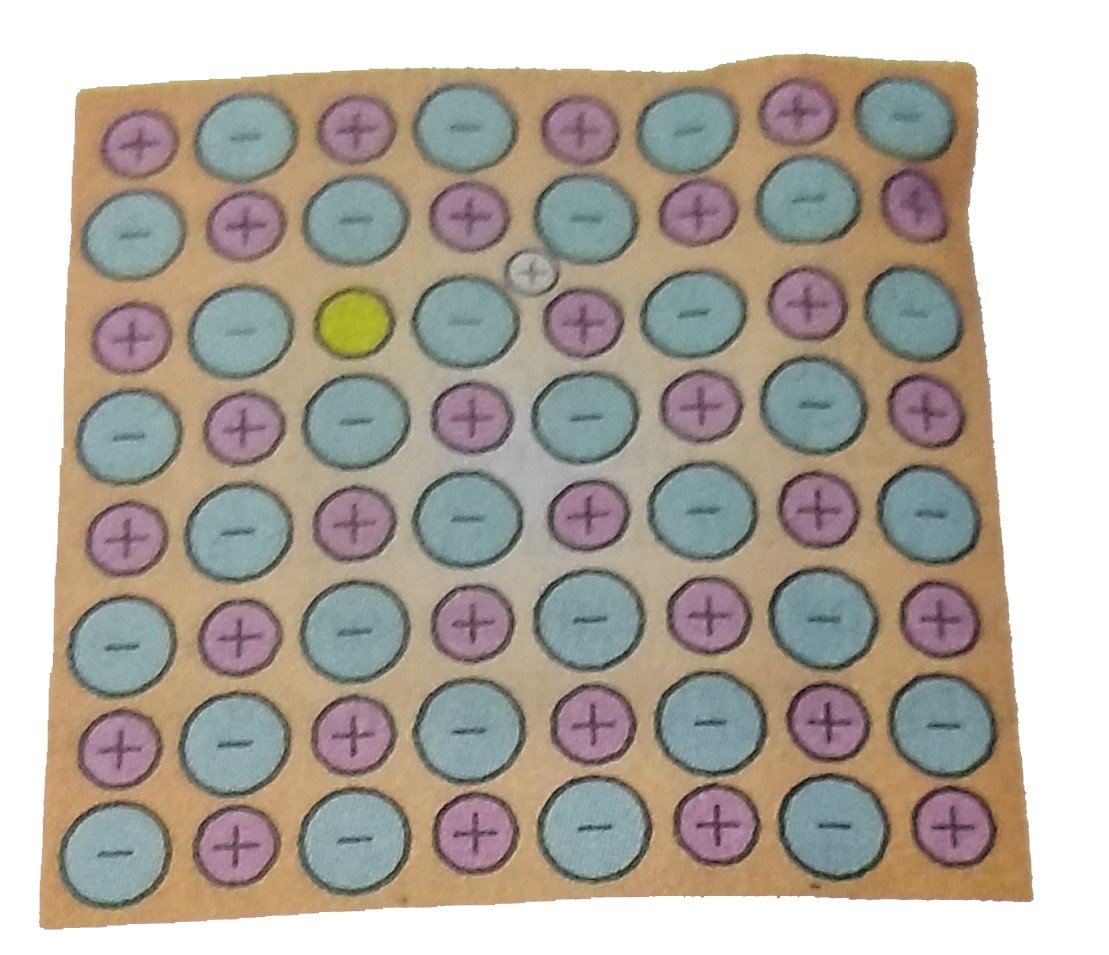
View SolutionConsider the figure of a defective crystal :
(a) Name the defect shown by this diagram.
(b) What is the effect of this defect on the solid ?
(c) Name as ionic compound which can show this type of defect.
View SolutionState True or False Schottky defects lower the density of related solids
View Solution

