Which of the following would be the best synthesis of benzoic acid from bromobenzene?
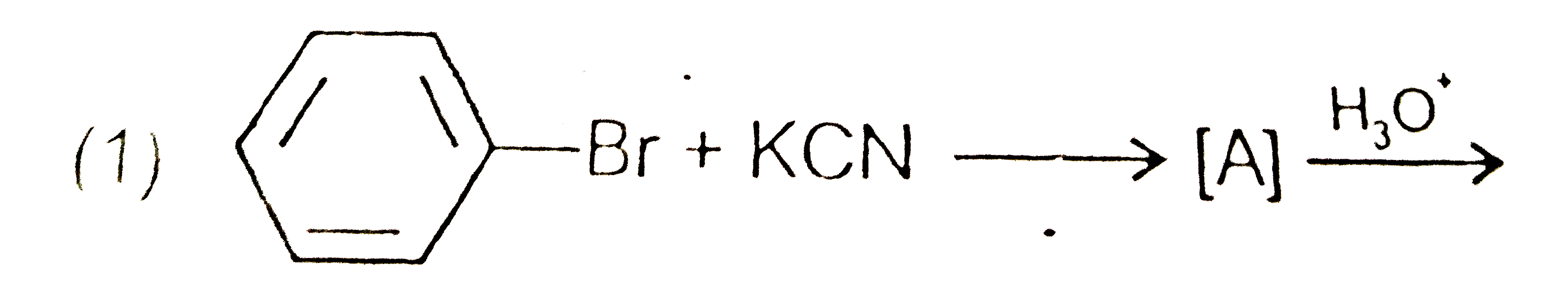
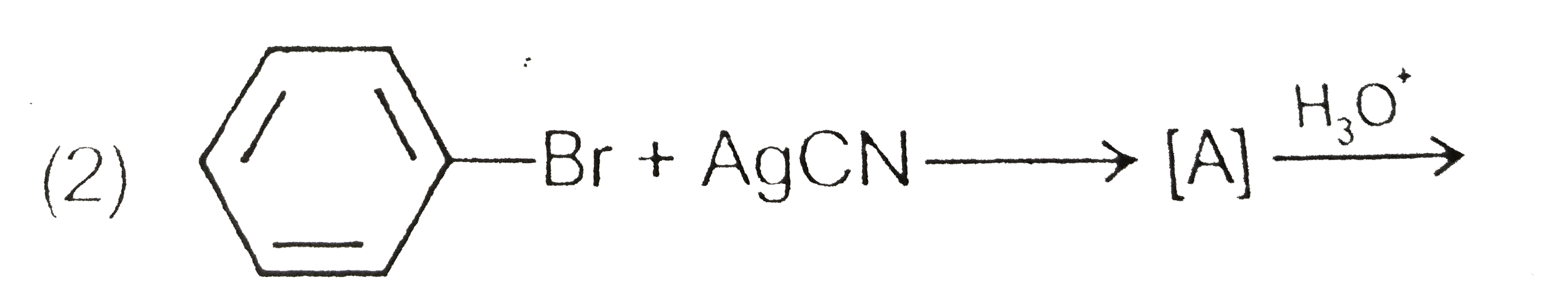
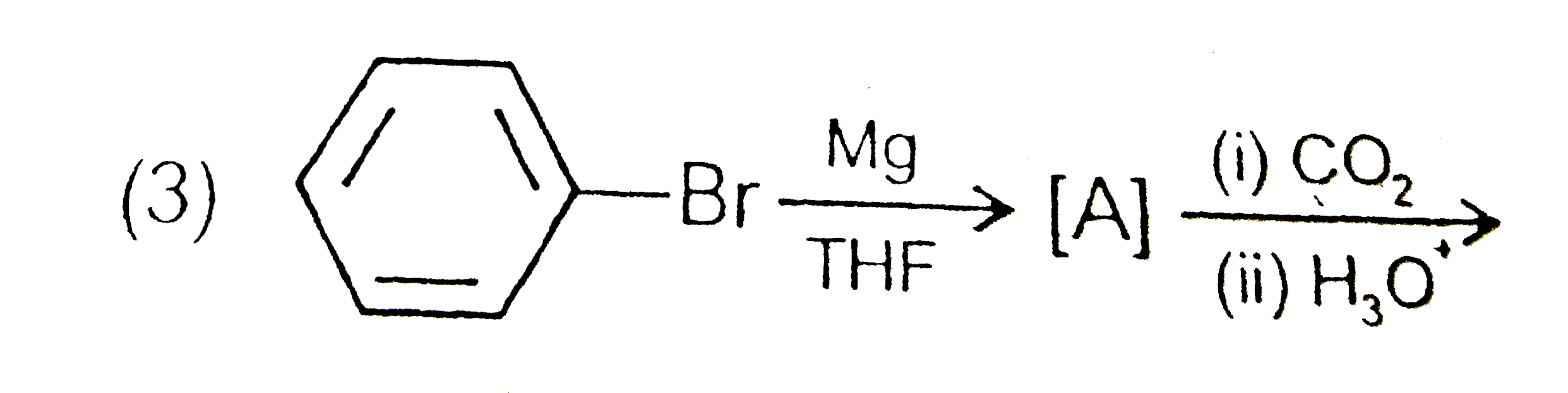
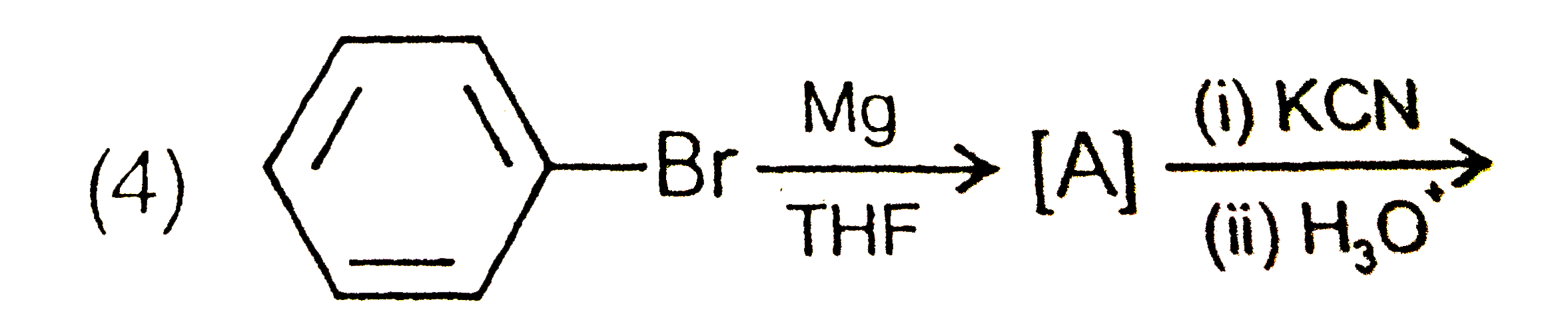
The correct Answer is:C
To synthesize benzoic acid from bromobenzene, we can analyze the provided reactions step by step. Here’s a detailed breakdown of the best synthesis method:
Step 1: Understanding the Reactants
- Bromobenzene is our starting material. It is an aryl halide, meaning it has a bromine atom attached to a benzene ring.
- We want to convert this compound into benzoic acid, which is a carboxylic acid with the formula C6H5COOH.
Step 2: Evaluating the Reactions
1. First Reaction: Bromobenzene + KCN
- This reaction involves a nucleophilic substitution where cyanide (CN⁻) acts as the nucleophile.
- The bromine atom is replaced by the cyanide group, forming benzonitrile (C6H5CN).
- Hydrolysis of benzonitrile leads to the formation of benzoic acid.
- Limitations: Aryl halides like bromobenzene do not favor nucleophilic substitution reactions, making this method less efficient.
2. Second Reaction: Bromobenzene + AgCN
- Similar to the first reaction, AgCN also leads to the formation of benzonitrile.
- Hydrolysis again yields benzoic acid.
- Limitations: The reaction with silver cyanide is also slow due to the nature of the metal, making it less favorable.
3. Third Reaction: Bromobenzene + Mg (in THF)
- Here, bromobenzene reacts with magnesium to form a Grignard reagent (C6H5MgBr).
- The Grignard reagent then reacts with carbon dioxide (CO2), which acts as an electrophile.
- The carbon from CO2 is attacked by the negatively charged carbon in the Grignard reagent, leading to the formation of benzoic acid upon hydrolysis.
- Advantages: This method is efficient, as Grignard reagents readily react with CO2, and the process is fast.
4. Fourth Reaction: Bromobenzene + Grignard Reagent + KCN
- This reaction also starts with the formation of a Grignard reagent.
- However, the subsequent reaction with KCN and hydrolysis is slower compared to the reaction with CO2.
- Limitations: The presence of KCN slows down the process, making this method less preferable.
Step 3: Conclusion
After evaluating all four reactions, the third reaction (bromobenzene with magnesium followed by CO2) is the best synthesis method for producing benzoic acid due to its efficiency and speed.
Final Answer
The best synthesis of benzoic acid from bromobenzene is through the third reaction: Bromobenzene + Mg (in THF) → Grignard Reagent → CO2 → Benzoic Acid.
---
- Bromobenzene is our starting material. It is an aryl halide, meaning it has a bromine atom attached to a benzene ring.
- We want to convert this compound into benzoic acid, which is a carboxylic acid with the formula C6H5COOH.
1. First Reaction: Bromobenzene + KCN
- This reaction involves a nucleophilic substitution where cyanide (CN⁻) acts as the nucleophile.
- The bromine atom is replaced by the cyanide group, forming benzonitrile (C6H5CN).
- Hydrolysis of benzonitrile leads to the formation of benzoic acid.
- Limitations: Aryl halides like bromobenzene do not favor nucleophilic substitution reactions, making this method less efficient.
- Similar to the first reaction, AgCN also leads to the formation of benzonitrile.
- Hydrolysis again yields benzoic acid.
- Limitations: The reaction with silver cyanide is also slow due to the nature of the metal, making it less favorable.
- Here, bromobenzene reacts with magnesium to form a Grignard reagent (C6H5MgBr).
- The Grignard reagent then reacts with carbon dioxide (CO2), which acts as an electrophile.
- The carbon from CO2 is attacked by the negatively charged carbon in the Grignard reagent, leading to the formation of benzoic acid upon hydrolysis.
- Advantages: This method is efficient, as Grignard reagents readily react with CO2, and the process is fast.
- This reaction also starts with the formation of a Grignard reagent.
- However, the subsequent reaction with KCN and hydrolysis is slower compared to the reaction with CO2.
- Limitations: The presence of KCN slows down the process, making this method less preferable.
After evaluating all four reactions, the third reaction (bromobenzene with magnesium followed by CO2) is the best synthesis method for producing benzoic acid due to its efficiency and speed.
The best synthesis of benzoic acid from bromobenzene is through the third reaction: Bromobenzene + Mg (in THF) → Grignard Reagent → CO2 → Benzoic Acid.
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:AAKASH INSTITUTE ENGLISHChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:Assignment (SECTION -C Objective type questions more than one options are correct)
Explore 11 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:AAKASH INSTITUTE ENGLISHChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:Assignment (SECTION -D Linked Comprehension type Questions)
Explore 6 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:AAKASH INSTITUTE ENGLISHChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:Assignment (Section A Competition Level Questions)
Explore 60 Videos
- ALCOHOLS, PHENOLS AND ETHERS Book:AAKASH INSTITUTE ENGLISHChapter:ALCOHOLS, PHENOLS AND ETHERS Exercise:Try Yourself
Explore 5 Videos
- AMINES Book:AAKASH INSTITUTE ENGLISHChapter:AMINES Exercise:Assignment Section -J (Aakash Challengers Questions)
Explore 9 Videos
Similar Questions
Knowledge Check
Which of the following would be the best synthesis of 2, 2-dimethylpropanoic acid?
A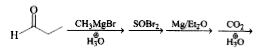 B
B C
C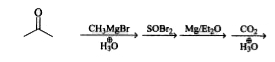 D
D
Which is the best synthesis of
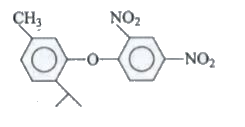 A
A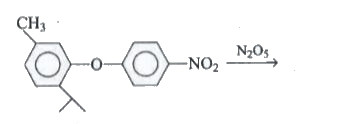 B
B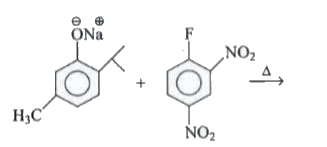 C
C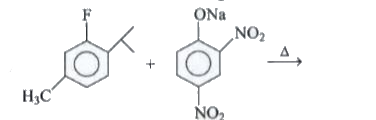 Dall are incorrect
Dall are incorrectWhich is best synthesis of
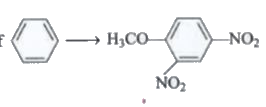 ABr2−−→feBr3HNO3−−−→H2SO4HNO3−−−→H2SO4CH3ONa−−−−−→CH3OHBHNO3−−−→H2SO4HNO3−−−→H2SO4Br2−−−→FeBr3CH3ONa−−−−−→CH3OHCHNO3−−−→H2SO4Br2−−−→FeBr3HNO3−−−→H2SO4CH3ONa−−−−−→CH3OHDHNO3−−−→H2SO4Br2−−−→FeBr3NaOCH3−−−−−→CH3OHHNO3−−−→H2SO4
ABr2−−→feBr3HNO3−−−→H2SO4HNO3−−−→H2SO4CH3ONa−−−−−→CH3OHBHNO3−−−→H2SO4HNO3−−−→H2SO4Br2−−−→FeBr3CH3ONa−−−−−→CH3OHCHNO3−−−→H2SO4Br2−−−→FeBr3HNO3−−−→H2SO4CH3ONa−−−−−→CH3OHDHNO3−−−→H2SO4Br2−−−→FeBr3NaOCH3−−−−−→CH3OHHNO3−−−→H2SO4
Which of the following is the best synthesis of the ether (A) shown below:
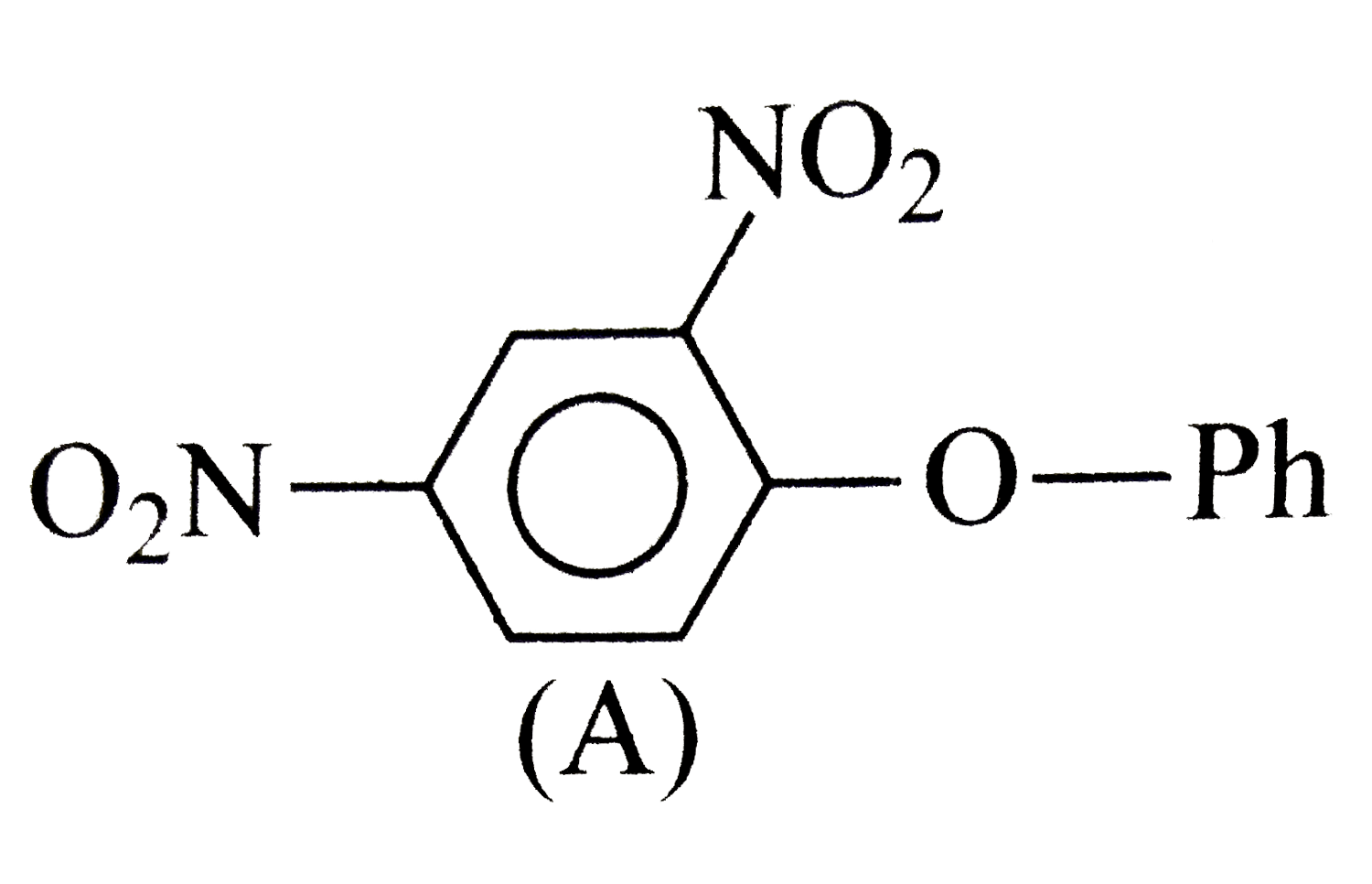
View SolutionWhich of the following statements are correct for benzoic acid?
View SolutionConversion of Benzoic acid to benzaldehyde
View SolutionWhich of the following reaction sequences would be the best for synthesis of 2-pentanone ?
View SolutionWhich of the following statements are correct for benzoic acid?
View Solution
