An organic compound [X]. C5H8O reacts with hydroxylamine to form [Y]. In the presence of conc. H2SO4 gives δ-lactam. [X] neither give Benedicts test nor it respond positively towards haloform test. The compound [X] is
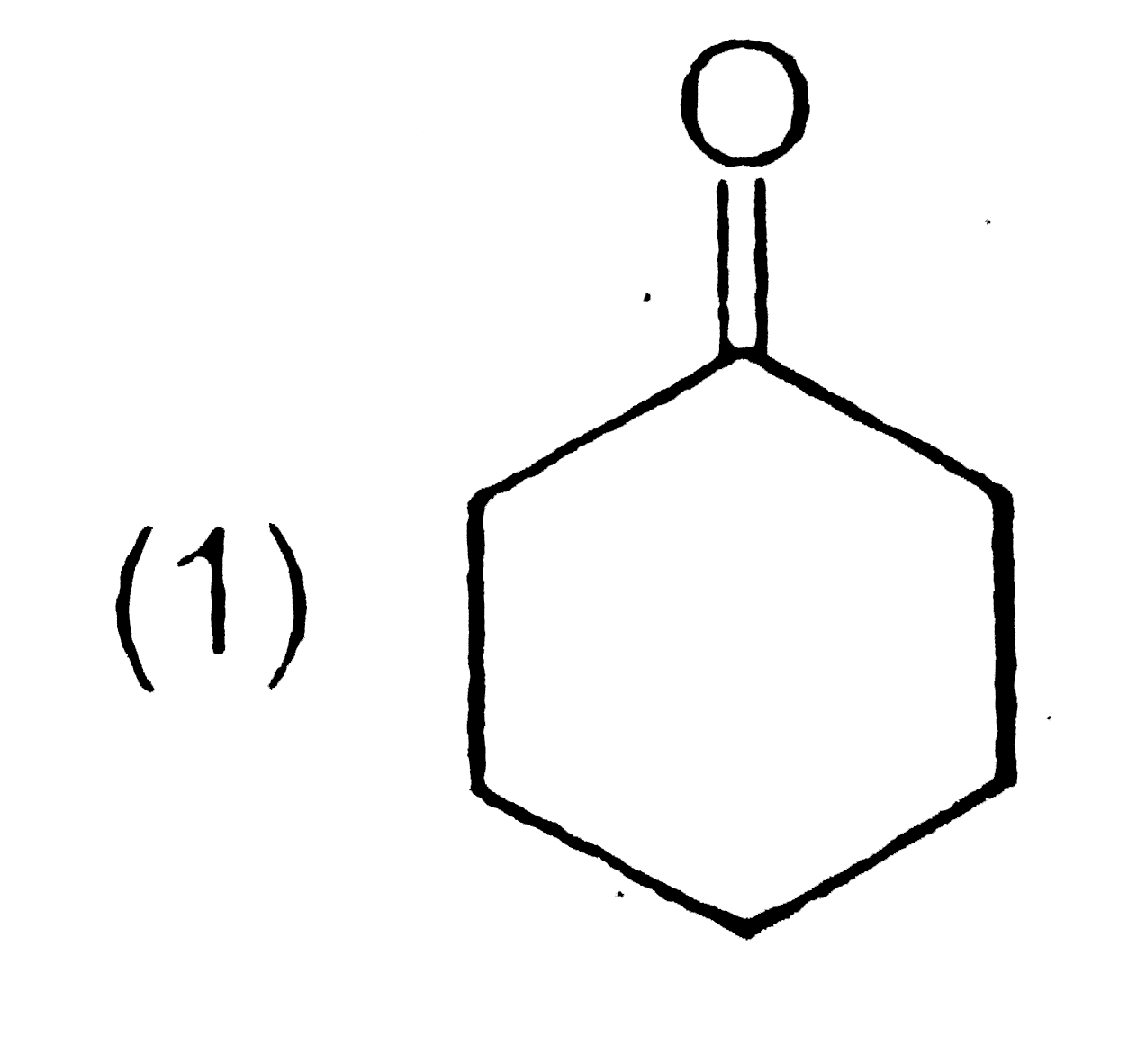
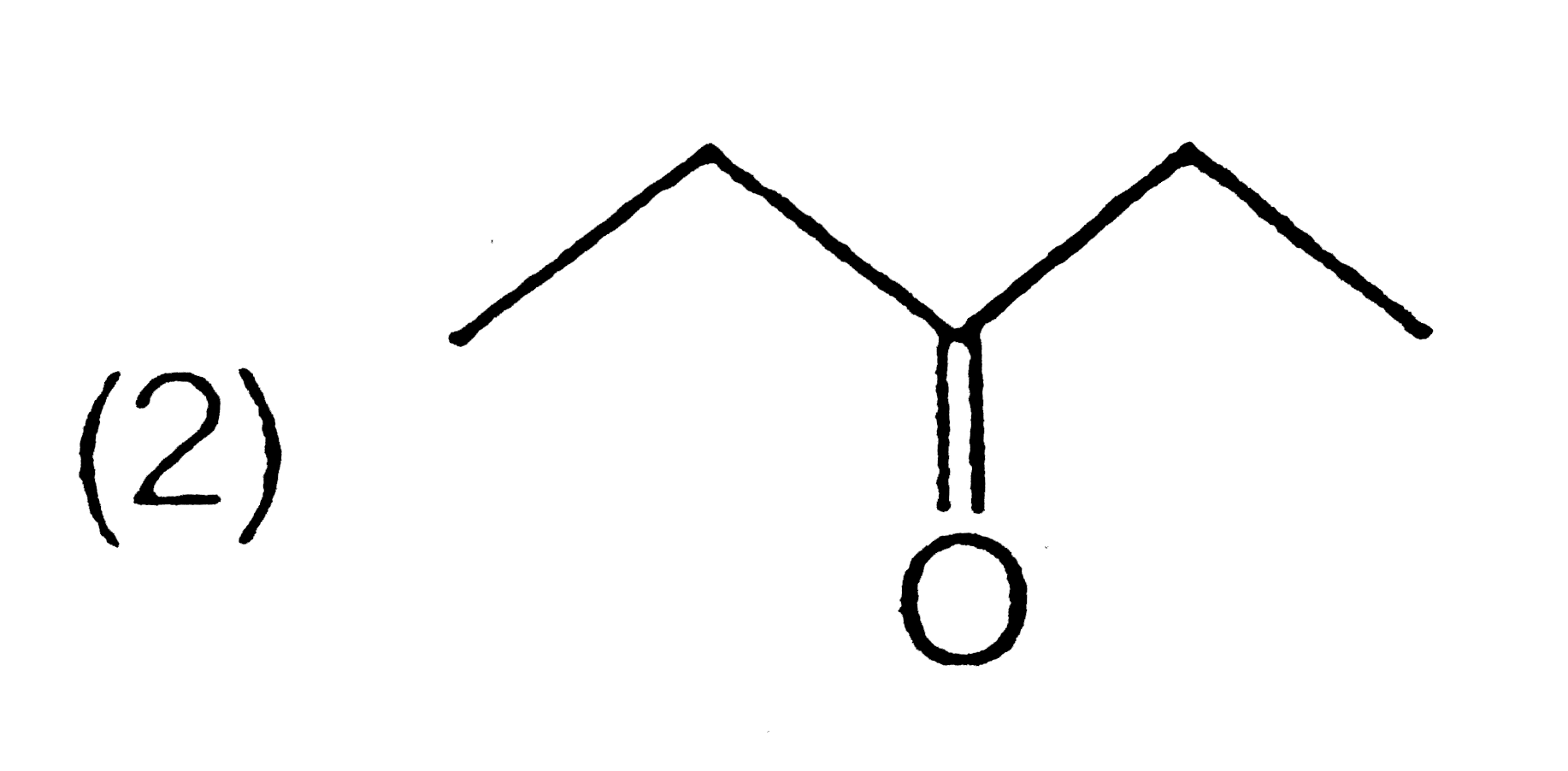
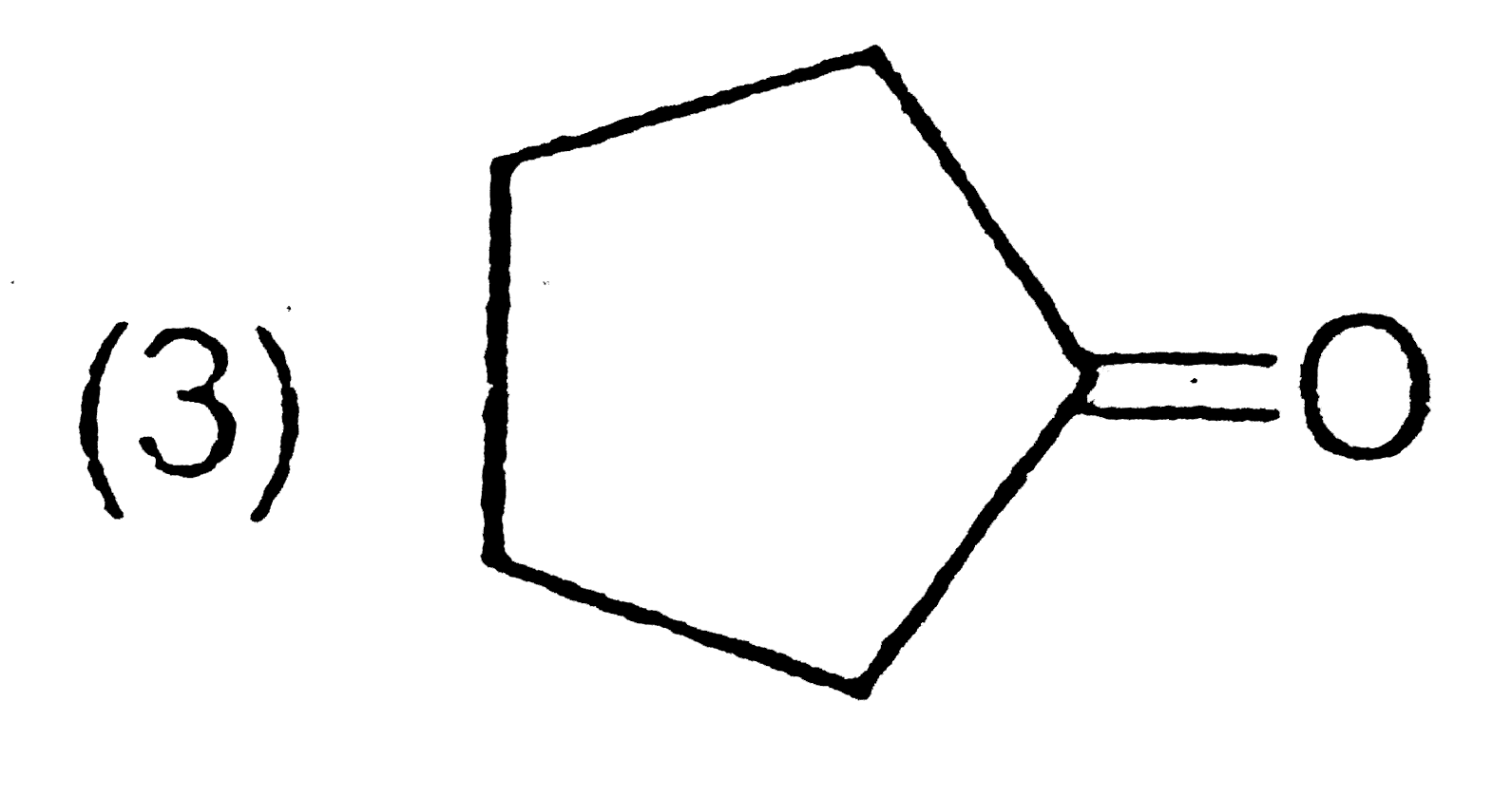
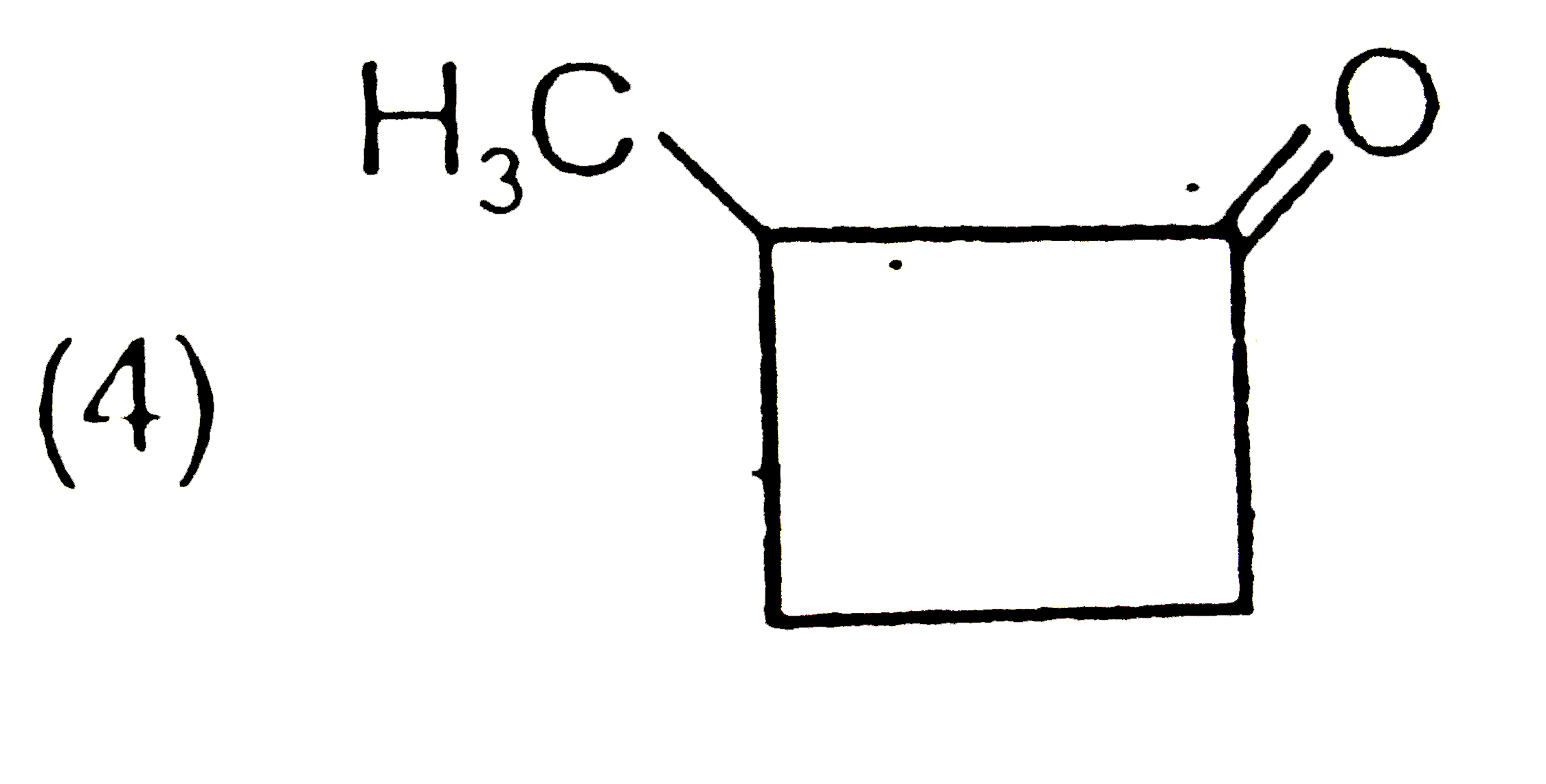
The correct Answer is:C
To solve the problem, we need to identify the organic compound (X) with the molecular formula C5H8O based on the given reactions and tests. Let's break it down step by step.
Step 1: Analyze the Molecular Formula
The compound (X) has the molecular formula C5H8O. This indicates that it could be an unsaturated compound, possibly an aldehyde or ketone due to the presence of a carbonyl group (C=O).
Hint: The degree of unsaturation can be calculated using the formula: Degree of Unsaturation=(2C+2−H)2.
Step 2: Reactivity with Hydroxylamine
The problem states that (X) reacts with hydroxylamine (NH2OH) to form (Y). This indicates that (X) contains a carbonyl group (either an aldehyde or a ketone) since hydroxylamine forms oximes with carbonyl compounds.
Hint: Hydroxylamine reacts with carbonyl compounds to form oximes, which are characterized by the functional group -C=N-OH.
Step 3: Formation of Delta-Lactam
In the presence of concentrated sulfuric acid (H2SO4), (X) gives a delta-lactam. A lactam is a cyclic amide, and a delta-lactam specifically refers to a lactam where the nitrogen atom is four carbons away from the carbonyl carbon.
Hint: A delta-lactam can be formed from a compound that has a suitable structure to allow for cyclization, typically involving a carbonyl group and an amine.
Step 4: Tests for Aldehyde and Ketone
The compound (X) does not give a positive Benedict's test, which means it is not an aldehyde that can be oxidized to a carboxylic acid. Additionally, it does not respond positively to the haloform test, indicating that it is not a methyl ketone (which would have a structure like CH3CO-).
Hint: The absence of a positive haloform test suggests that there is no methyl ketone structure (CH3CO-) present in (X).
Step 5: Identify Possible Structures
Given the molecular formula C5H8O and the information from the tests, we can deduce that (X) is likely a cyclic compound since it can form a lactam. The options provided in the question likely include cyclopentanone or cyclobutanone derivatives.
Hint: Consider the structure of cyclopentanone and cyclobutanone to see which one fits the criteria of not giving the tests mentioned.
Step 6: Conclusion
After analyzing the possible structures, we find that cyclopentanone (which has a carbonyl group but does not fit the criteria for the tests mentioned) is the most plausible candidate for (X).
Thus, the compound (X) is cyclopentanone.
Final Answer
The compound (X) is cyclopentanone.
---
The compound (X) has the molecular formula C5H8O. This indicates that it could be an unsaturated compound, possibly an aldehyde or ketone due to the presence of a carbonyl group (C=O).
The problem states that (X) reacts with hydroxylamine (NH2OH) to form (Y). This indicates that (X) contains a carbonyl group (either an aldehyde or a ketone) since hydroxylamine forms oximes with carbonyl compounds.
In the presence of concentrated sulfuric acid (H2SO4), (X) gives a delta-lactam. A lactam is a cyclic amide, and a delta-lactam specifically refers to a lactam where the nitrogen atom is four carbons away from the carbonyl carbon.
The compound (X) does not give a positive Benedict's test, which means it is not an aldehyde that can be oxidized to a carboxylic acid. Additionally, it does not respond positively to the haloform test, indicating that it is not a methyl ketone (which would have a structure like CH3CO-).
Given the molecular formula C5H8O and the information from the tests, we can deduce that (X) is likely a cyclic compound since it can form a lactam. The options provided in the question likely include cyclopentanone or cyclobutanone derivatives.
After analyzing the possible structures, we find that cyclopentanone (which has a carbonyl group but does not fit the criteria for the tests mentioned) is the most plausible candidate for (X).
The compound (X) is cyclopentanone.
Topper's Solved these Questions
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Assignment (SECTION -C Objective type questions more than one options are correct)
11 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Assignment (SECTION -D Linked Comprehension type Questions)
6 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDS EXERCISE - Assignment (Section A Competition Level Questions)
60 Videos
- ALCOHOLS, PHENOLS AND ETHERS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - Try Yourself
5 Videos
- AMINES BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - AMINES EXERCISE - Assignment Section -J (Aakash Challengers Questions)
9 Videos
Similar Questions
Knowledge Check
Phenol reacts with conc. HNO3 in the presence of conc. H2SO4 to give :
Ameta nitrophenolBortho nitrophenolCortho and para nitrophenolDpicric acidPhenol reacts with acetone in the presence of conc. H2SO4 to from a C15H16O2 product. Which of the following compound is this product ?
A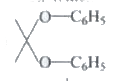 B
B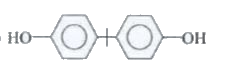 C
C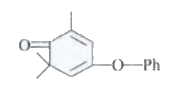 D
D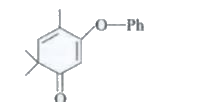
Compound (X) C4H8O, which reacts with 2,4-DNP derivative and gives negative haloform test is :
ACH3−O∣∣C−CH2−CH3BCH3−CH∣CH3−CHOC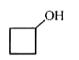 DCH3−CH2−OH∣CH−CH3
DCH3−CH2−OH∣CH−CH3
An organic compound A (C2H6O) reacts with sodium to form a compound B with the evolution of H2 and gives a yellow compound C on reacting with iodine and NaOH. When heated with conc. H2SO4 at 413 K, it gives a compound D (C4H10O) which on reaction wiht conc. HI at 373 K gives compound E. The compound D is also obtained when B is heated with E. Idntify the compounds A,B,C,D and E write the equations for the reaction involved.
View SolutionWhich one of the following compound gives aspirin on reacting with acetic anhydride in presence of H2SO4
View Solution(i) An organic compound A with molecular formula C7H8 on oxidation by chromyl chloride in the presence of CCl4 gives a compound B which gives positive Tollen's test. The compound B on treatment with NaOH followed by acid hydrolysis gives two product C and D. C on oxidation gives B which on further oxidation gives D. the compound D on distillation with sodalime gives a hydrocarbon E. Below 60∘C, concentrated nitric acid reacts with E in the presence of concentrated sulphuric acid forming a compound F. identify the compounds A,B,C,D,E and F.
(ii) Give chemical test to distinguish : Formaldehyde and acetaldehyde.
View SolutionCompound X C4H8O which gives 2,4-DNP derivative and negative iodoform test is
View SolutionCompound X C4H8O which gives 2,4-DNP derivative and negative iodoform test is
View Solution

