if work is done by the system then W is
Positive
Negative
Zero
Depends upon the thermodynamic process
The correct Answer is:A
Positive
Topper's Solved these Questions
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - EXERCISE
20 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - ASSIGNMENT (SECTION -A) (Objective Type Questions)
47 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMODYNAMICSEXERCISE - Assignment (Section-J) Aakash Challengers Questions
6 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - Assignment (Section-J) Akash Challengers Questions
7 Videos
- UNITS AND MEASUREMENTSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - UNITS AND MEASUREMENTSEXERCISE - ASSIGNMENT (SECTION - D)
15 Videos
Similar Questions
When are the two bodies in thermal equilibrium?
View SolutionIf W si work done by the system (−ve), the mathematical respresentation of the first law of thermodynamics is//are
View SolutionThe given figrue shows the variation of force exerted on the piston by an ideal gas (enclosed in a piston-cylinder arrangement) that undergoes a process during which piston position changes from 0.1 to 0.4 m. if the initial internal energy of the system is 3.25 J then find the net heat (in joule) absorbed during the process.
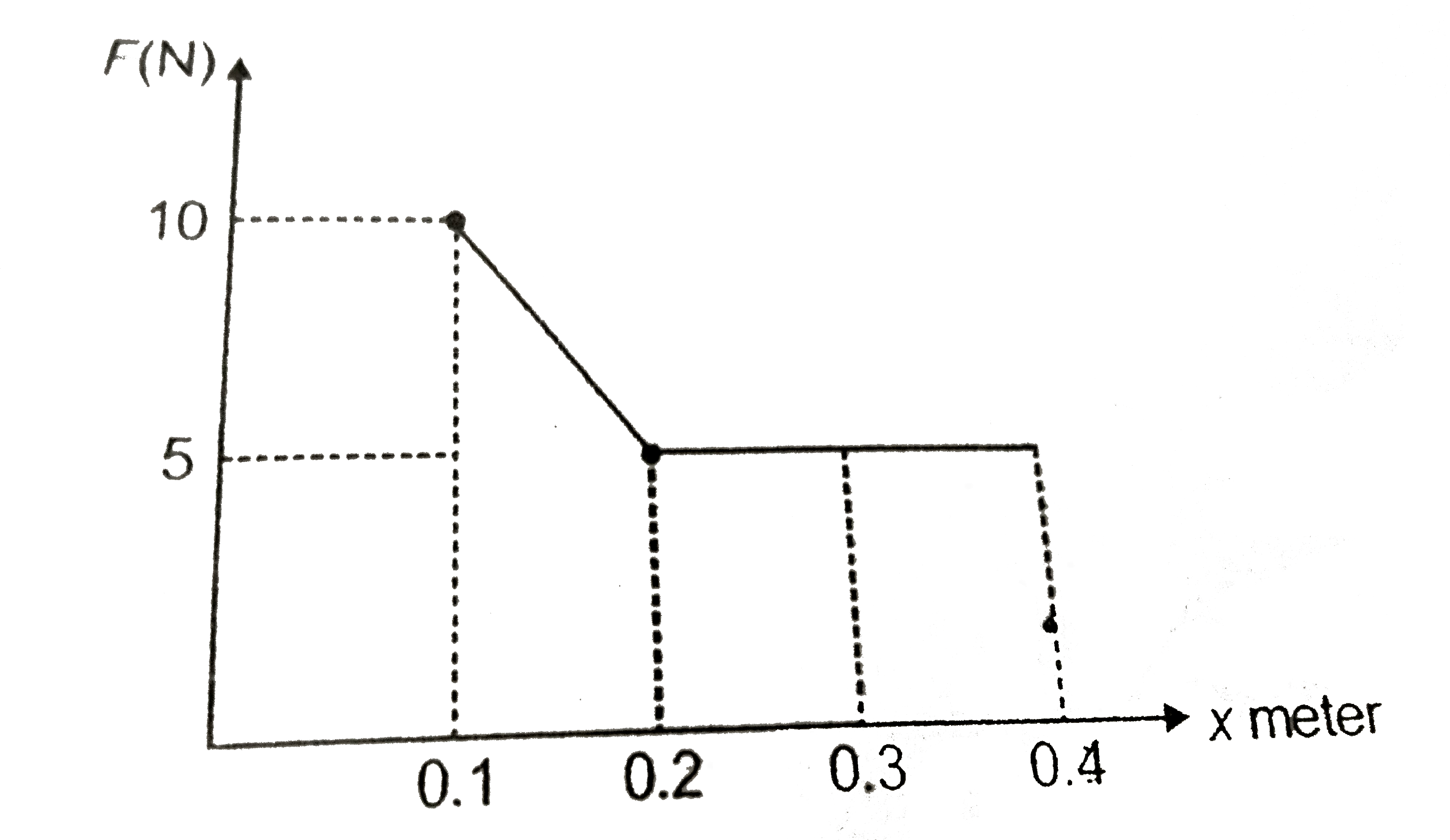
View SolutionA slab of cunductivity k is in the shape of trapezoid as shown I figure. If the two end faces be maintained at temperature T1=50∘CandT2=25∘C, then determine the thermal current through the slab by ignoring any heat loss through the lateral surfaces.
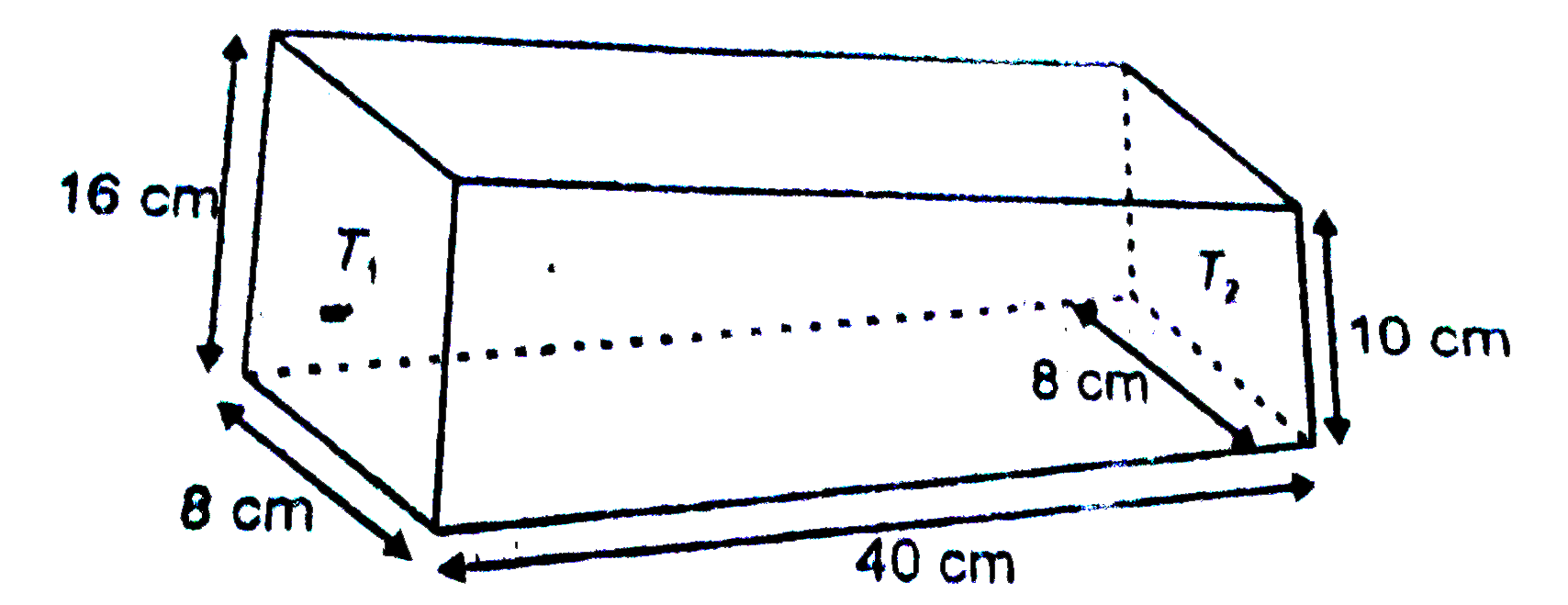 .
.
View SolutionA : PARSEC and light year, both measure time
R : Both have dimension of time.
View Solutionw is positive when the work is done on the system.
View SolutionIf q is the amount of heat absorbed by the system and W the amount of work done on the system (+ve) , the change in the energy of the system is given by:
View SolutionWhen no heat is absorbed by the system them from the surroundings, but work (w) is done on the system, the change in internal energy of the system is given as
View SolutionIf W is the amount of work done by the system and q is the amount of heat supplied to the system, identify the type of the system
View Solutionif work is done by the system then W is
View Solutionजब कार्य तन्त्र द्वारा किया जाता है तब w का मान ………. होता है ।
View SolutionIf W is the amoung of work done by the system and q is the amount of heat supplied to the system, identify the type of the system.
View SolutionNo heat is absorbed by the system from the surroundings, but work (w) is done on the system. What type of wall does the system have?
View SolutionWhen work (w) is done by the system, the sign of w is negative. But when it is done on the system, the sign of w is positive, give reason.
View SolutionIn a process, a system releases 500J of heat and work done on the system is 300J. In the process, q=____ and w=_____.
View SolutionIn a process, if the heat released by the system and work done on the system are 90 J and 120 J, respectively,, then what will be of q and w?
View Solution

