In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the gas will be:
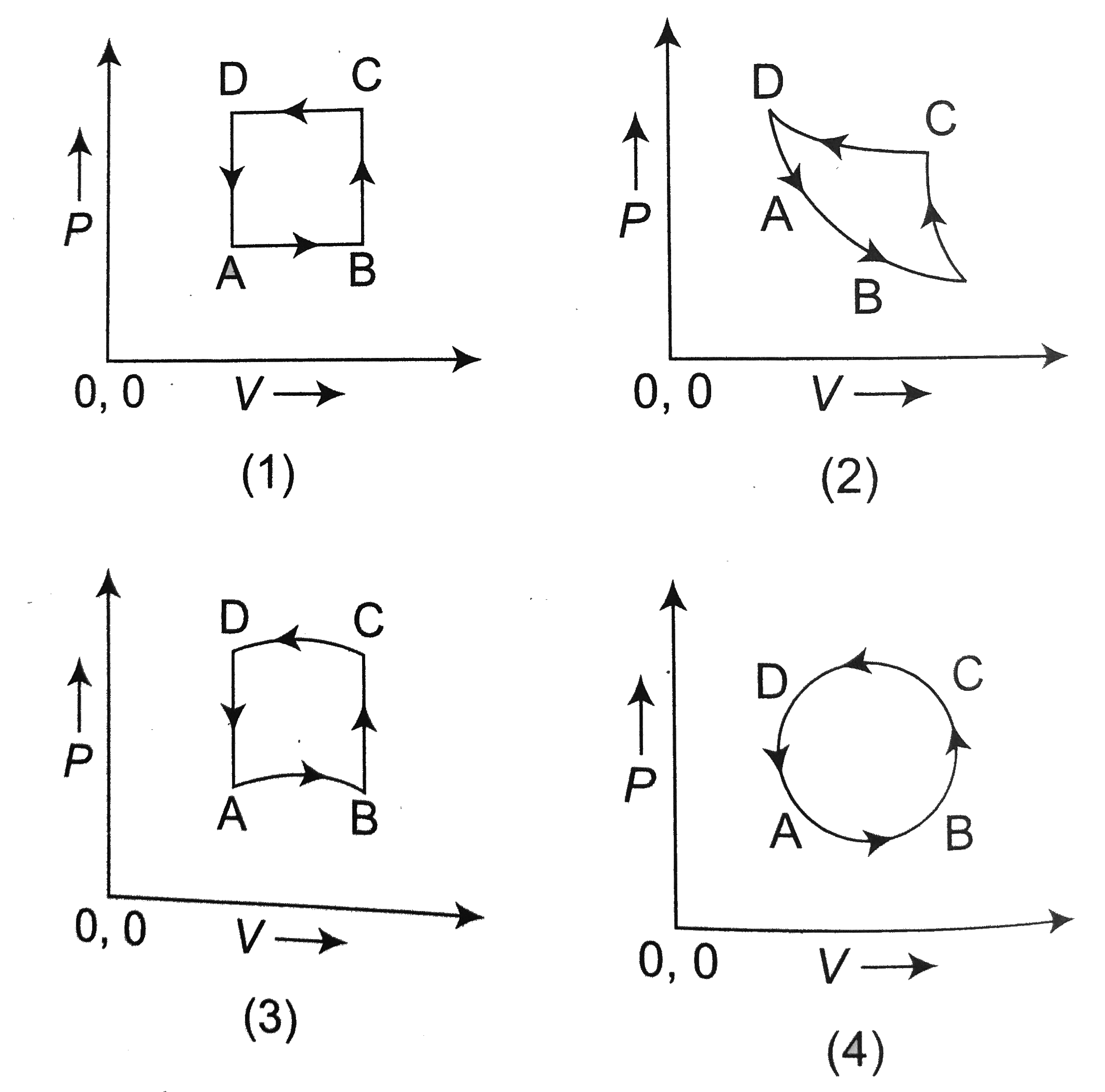
Positive in all cases from (a) to (d)
Positive in cases (a), (b) and (c ) but zero in case (d)
Negative in cases (a), (b) and (c ) but zero in case (d)
Zero in all the four cases
The correct Answer is:D
Step by step video solution for In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the gas will be: by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBook:AAKASH INSTITUTE ENGLISHChapter:THERMODYNAMICSExercise:ASSIGNMENT (SECTION -B) (Objective Type Questions)
Explore 30 Videos
- THERMODYNAMICSBook:AAKASH INSTITUTE ENGLISHChapter:THERMODYNAMICSExercise:ASSIGNMENT (SECTION -C) (Previous Year Questions)
Explore 63 Videos
- THERMODYNAMICSBook:AAKASH INSTITUTE ENGLISHChapter:THERMODYNAMICSExercise:EXERCISE
Explore 20 Videos
- THERMAL PROPERTIES OF MATTERBook:AAKASH INSTITUTE ENGLISHChapter:THERMAL PROPERTIES OF MATTERExercise:Assignment (Section-J) Akash Challengers Questions
Explore 7 Videos
- UNITS AND MEASUREMENTSBook:AAKASH INSTITUTE ENGLISHChapter:UNITS AND MEASUREMENTSExercise:ASSIGNMENT (SECTION - D)
Explore 15 Videos
Similar Questions
In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the tgas will be:

View SolutionChoose the correct option:
In following figs. Variation of volume by change of pressure is shown in Fig. A gas is taken along the path ABCDA. The change in internal energy of the the gas will be:

View Solution
Knowledge Check
If a gas is comprssed adiabatically by doing work of 150 J the change in internal energy of the gas is
Aa. 100 JBb. 150 JCc. 200 JDd. 250 J
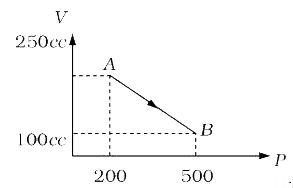
A mono-atomic gas is taken along path AB as shown. Calculate change in internal energy of the gas.
View SolutionTwo moles of helium gas are taken along the path ABCD (as shown in Fig.) The work done by the gas is

View SolutionTwo moles of helium gas are taken along the path ABCD (as shown in Fig.) The work done by the gas is

View SolutionWhat happens to the change in internal energy of a gas during adiabatic expansion?
View SolutionBy which methods can the internal energy of an ideal gas be changed ?
View Solution400 J of work is done on a gas to reduce its volume by compression adiabatically. What is the change in internal energy of the gas?
View SolutionA gas of volume changes 2 litre to 10 litre at constant temperature 300K, then the change in internal energy will be :
View Solution
