Arrange the following in decreasing order of acidic nature of
(a) (i) F3CCH2OH , (ii) (CH3)3CCH2OH , (iii) FCH2CH2C2OH
(b)
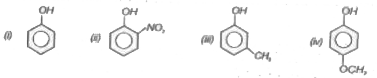
(a) (i)>(iii)>(ii)
(b) (ii)>(i)>(iii)>(iv)
−NO2 is stabilizing 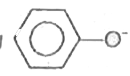 by strong electron withdrawing nature, −CH3 is destabilizing by electron donating nature (+ 1) and −OCH3 is also destabilizing due to electron donting nature (+ R , - 1)
by strong electron withdrawing nature, −CH3 is destabilizing by electron donating nature (+ 1) and −OCH3 is also destabilizing due to electron donting nature (+ R , - 1)
Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - Try yourself
5 Videos
- ALCOHOLS, PHENOLS AND ETHERSBOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALCOHOLS, PHENOLS AND ETHERSEXERCISE - Exercise
40 Videos
- ALCOHOLS, PHENOLS AND ETHERS BOOK - AAKASH INSTITUTE ENGLISHCHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - Try Yourself
5 Videos
Similar Questions
The pH value of decinormal solution of NH4OH, which is 20% ionised, is:
View SolutionKetones can be converted to tertiary alcohols by
View SolutionFind the product of given reaction
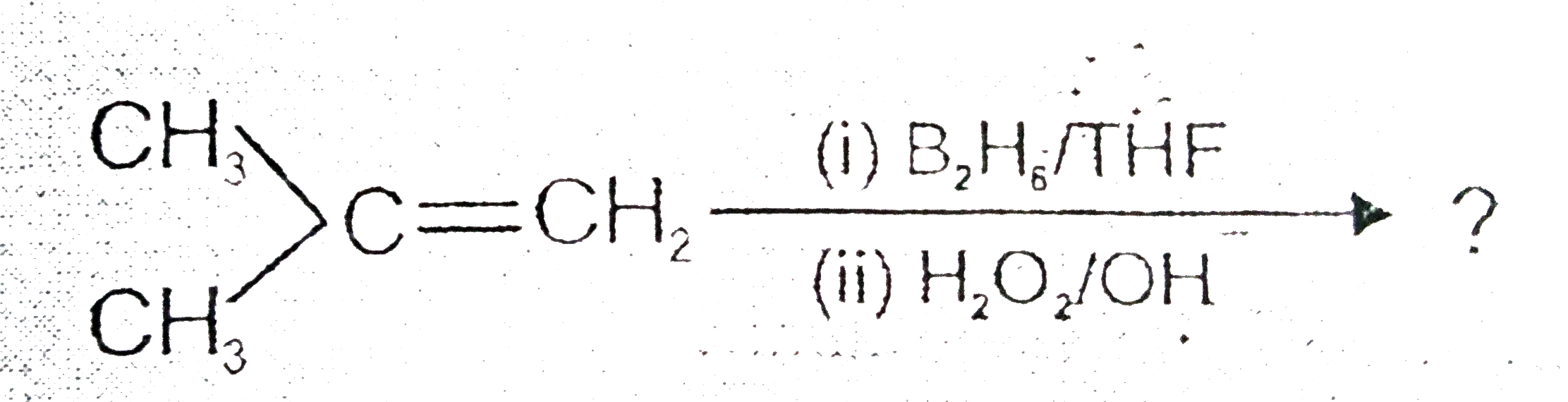
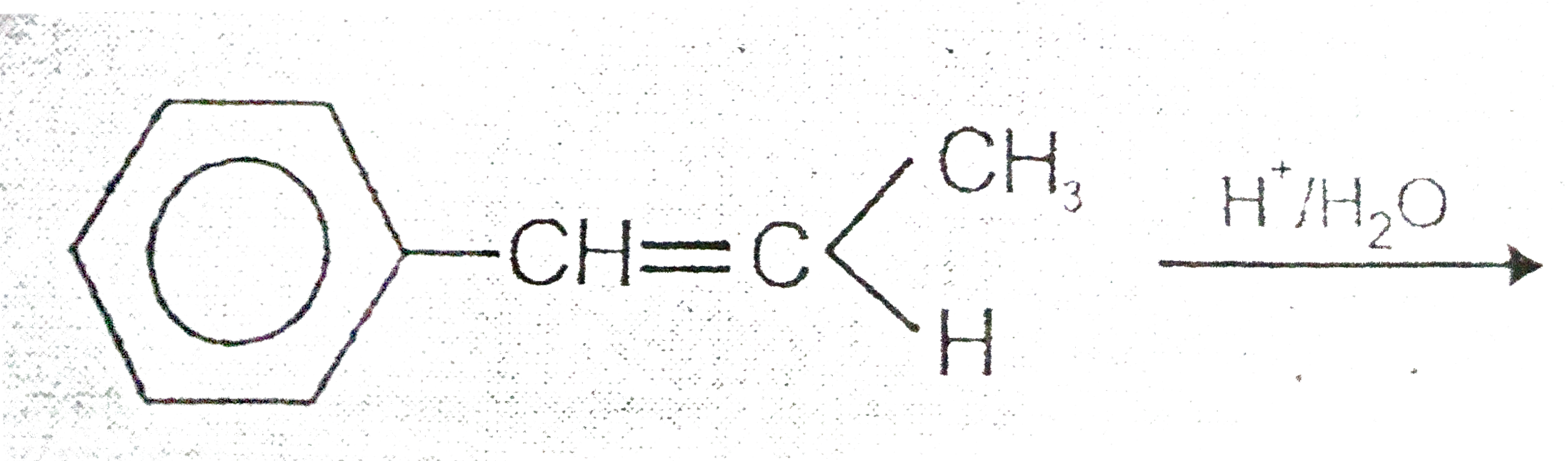

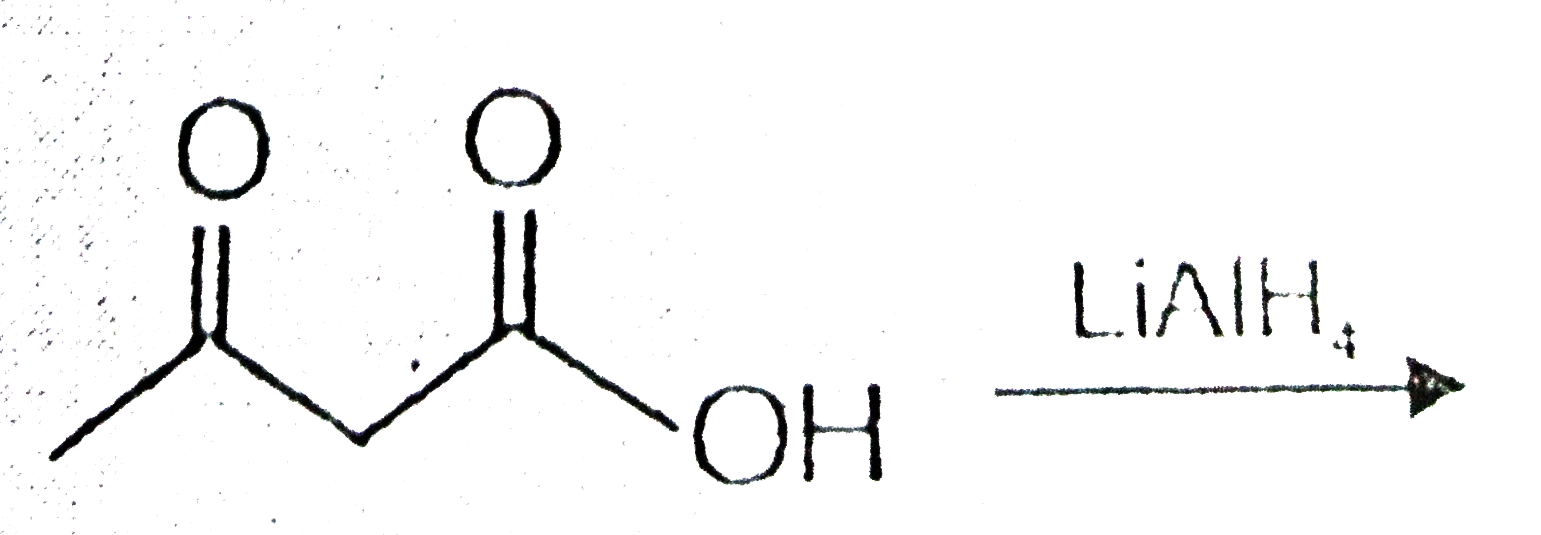
View SolutionArrange the following compounds in decreasing order of acidity :
CH3CH2OH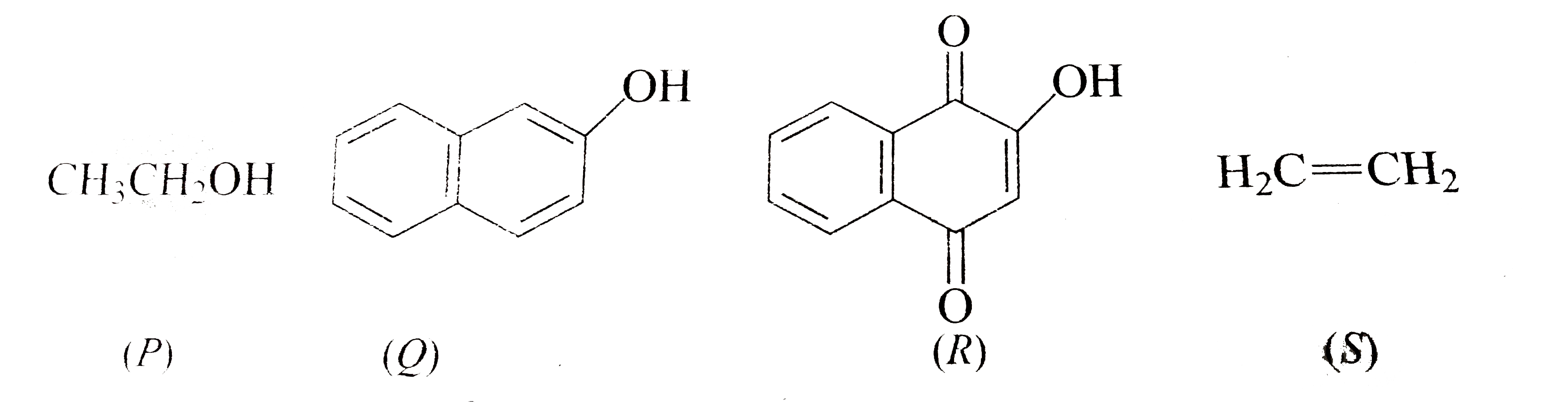
H2C=CH2
View SolutionClassify the following as primary, secondary and tertiary alcohols: (i) CH_(3)-overset(CH_(3))overset("| ")underset(CH_(3))underset("| ")(C )-CH_(2)OH (ii) H_(2)C=CH-CH_(2)OH (iii) CH_(3)-CH_(2)-CH_(2)-OH (iv)
View SolutionClassify the following into primary, secondary and tertiary alcohols (i) CH-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-CH_(2)OH , (ii) H_(2)C=CH-CH_(2)OH , (iii) CH_(3)-CH_(2)-CH_(2)OH
View SolutionArrange the following in decreasing order of solubility in water
(i) CH3CH2OH (ii) CH3OH (iii) C6H5CH2OH.
View SolutionHow many bonds (sigma and pi ) are there in the following molecules? (i) H_(2)C=C=CH_(2) (ii) CH_(3)-C-=CH (iii) H_(2)C=CH-C-=N (iv) CH_(3)NO_(2) (v) CH_(3)OH (iv) C_(2)H_(2)
View SolutionArrange the following acid in decreasing order of their acidic strength : (I) CH_(3)-OH (II) CH_(3)CH_(2)-OH (III) CH_(3)-underset(CH_(3))underset(|)(CH)-OH (IV) CH_(3)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-OH
View Solutionनिम्नलिखित को कारण बताते हुए दिये गए क्रम में व्यवस्थित कीजिए-
(i)C2H5OH,C6H5CH2,OH,C6H5OH: जल में विलेयता के बढ़ते हुए क्रम में
(ii)(CH3)3CCH2OH(CH3)2CHCH2CH2OH,CH3(CH2)CH2OH, क्वथनांक के घटते हुए क्रम में ,
View SolutionThe order of reactivity of following alcohols with halogen acids is _____ (A). CH_(3)CH_(2)-CH_(2)-OH (B). CH_(3)CH_(2)-underset(CH_(3))underset(|)(C)H-OH (C). CH_(3)CH_(2)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-OH
View SolutionGive the IUPAC name of each of the following and classify them as 1∘,2∘ and 3∘
(a) CH3(CH2)3CHOHCH(CH3)2 ,
(b) (CH3)3C−CH2OH
( c) (CH3)2C−C∣C6H5−OH
(d) BrCH3−C∣OHH2−CH−C(CH3)3
(e) CH2=CH−CHOHCH
(f) PhCH2OH
(g) HOCH2CH2CH2CH2C6H5
(h) (C2H5)2COH
View SolutionThe compound that reacts with NaOH . (A) CH_(3)OH (B) CH_(3)CH_(2)OH (C) C_(6)H_(5)OH (D) C_(6)H_(5)CH_(2)OH
View SolutionHow would you carry out the following conversions ? (a) H_(3)C CH_(2)=CH_(2) to H_(3)C- CH_(2)CH_(2)CH_(2)OH (b) H_(3)C CH_(2)-CH =CH_(2) to H_(3)C-CH_(2)-CH(OH)CH_(2)OH (c) H_(3)C C-= CH to H_(3)C COCH_(3) (d) Br_(2)CH CHBr_(2) to HC -=CH
View SolutionArrange the following in decreasing order of acidic nature of (a) (i) F_(3) C C H_(2) OH , (ii) (CH_(3))_(3) C C H_(2) OH , (iii) FCH_(2) CH_(2) C_(2) OH (b)
View SolutionClassify the following as primary, secondary and tertiary alcohols:
(i) CH3−CH3| C| CH3−CH2OH (ii) H2C=CH−CH2OH
(iii) CH3−CH2−CH2−OH
(iv)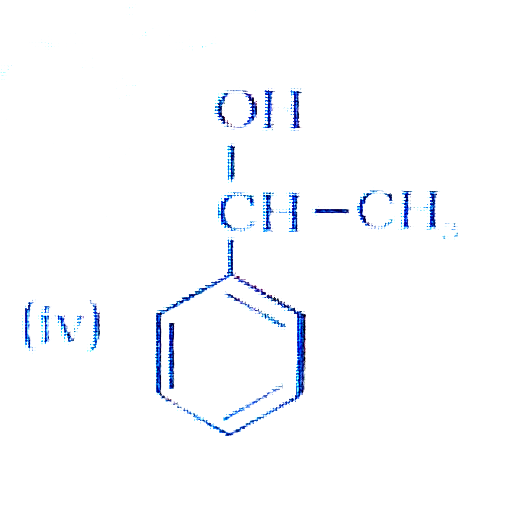
View Solution

