Ψ420 represents
4pz
4dz2
4s
5Px
The correct Answer is:B
To solve the question regarding what Ψ420 represents, we will break down the components of the notation step by step.
1. Understanding the Notation:
The notation Ψnlm represents a wave function in quantum mechanics, where:
- n is the principal quantum number,
- l is the angular momentum quantum number,
- m is the magnetic quantum number.
2. Identifying the Values:
From Ψ420, we can identify:
- \( n = 4 \)
- \( l = 2 \)
- \( m = 0 \)
3. Interpreting the Principal Quantum Number (n):
The principal quantum number \( n = 4 \) indicates that the electron is in the fourth energy level or shell.
4. Interpreting the Angular Momentum Quantum Number (l):
The angular momentum quantum number \( l = 2 \) corresponds to the type of orbital:
- \( l = 0 \) corresponds to an s orbital,
- \( l = 1 \) corresponds to a p orbital,
- \( l = 2 \) corresponds to a d orbital.
Therefore, \( l = 2 \) indicates that we are dealing with a d orbital.
5. Interpreting the Magnetic Quantum Number (m):
The magnetic quantum number \( m = 0 \) specifies the orientation of the orbital. For \( l = 2 \) (d orbitals), the possible values of \( m \) can be -2, -1, 0, +1, +2. The value \( m = 0 \) corresponds to the \( d_{z^2} \) orbital.
6. Conclusion:
Combining all the information:
- The principal quantum number \( n = 4 \) indicates the fourth shell.
- The angular momentum quantum number \( l = 2 \) indicates a d orbital.
- The magnetic quantum number \( m = 0 \) indicates the \( d_{z^2} \) orbital.
Thus, Ψ420 represents the 4d_z^2 orbital.
Final Answer:
Ψ420 represents the 4d_z^2 orbital.
The notation Ψnlm represents a wave function in quantum mechanics, where:
- n is the principal quantum number,
- l is the angular momentum quantum number,
- m is the magnetic quantum number.
From Ψ420, we can identify:
- \( n = 4 \)
- \( l = 2 \)
- \( m = 0 \)
The principal quantum number \( n = 4 \) indicates that the electron is in the fourth energy level or shell.
The angular momentum quantum number \( l = 2 \) corresponds to the type of orbital:
- \( l = 0 \) corresponds to an s orbital,
- \( l = 1 \) corresponds to a p orbital,
- \( l = 2 \) corresponds to a d orbital.
Therefore, \( l = 2 \) indicates that we are dealing with a d orbital.
The magnetic quantum number \( m = 0 \) specifies the orientation of the orbital. For \( l = 2 \) (d orbitals), the possible values of \( m \) can be -2, -1, 0, +1, +2. The value \( m = 0 \) corresponds to the \( d_{z^2} \) orbital.
Combining all the information:
- The principal quantum number \( n = 4 \) indicates the fourth shell.
- The angular momentum quantum number \( l = 2 \) indicates a d orbital.
- The magnetic quantum number \( m = 0 \) indicates the \( d_{z^2} \) orbital.
Ψ420 represents the 4d_z^2 orbital.
Topper's Solved these Questions
Similar Questions
The correct order of boiling points of isomeric amines is
View SolutionWhich of the following carbohydrate is a monosaccharide?
View SolutionWhich of the following represent represent clear electropositive properties
View Solutionयदि P, 7 को दर्शाता है, X, 9 को दर्शाता है, M. 5 को दर्शाता है, Z. 8 को दर्शाता हैL, 2 को और T, 1 को, तो ZLTPXM का मान क्या होगा ?
View SolutionIf natality is represented by -B If mortality is represented by -D if immigration is represented -I If emigration is represented by -E If population density at time t+1 is represented by
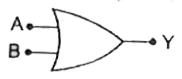
View Solutionचित्र में प्रदर्शित लॉजिक निकाय निरूपित करता है
View SolutionΨ420 represents
View Solutionयदि
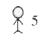 5 विद्यार्थियों को निरुपित करता है, तो
5 विद्यार्थियों को निरुपित करता है, तो 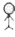 द्वारा निरुपित विद्यार्थियों की संख्या है :
द्वारा निरुपित विद्यार्थियों की संख्या है :
View Solutionयदि एक मानचित्र पर 4km को 1cm से निरूपित किया जाता है, तो 16km को _______cm से निरूपित किया जाएगा।
View SolutionIf
 represents 2 persons, then
represents 2 persons, then  represents _____ persons.
represents _____ persons.
View SolutionIf P represents 7, X represents 9, M represents 5, Z represents 8, L represents 2 and T represents 1, then what is the value of ZLTPXM?
View SolutionIf * represents 4 balls, No. of * to be drawn to represents 40 balls.
View SolutionIf one represents 35 pens, then will represent
View SolutionIf
 represents 150 pillars, what does
represents 150 pillars, what does 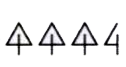 represents ?
represents ?
View SolutionIf
 represents 2 toffees, then
represents 2 toffees, then  represents how many toffees ?
represents how many toffees ?
View Solution

