Which of the following compound is possible :
PbI4
AlO2
NaF2
PbO2
The correct Answer is:D
To determine which of the given compounds is possible, we will analyze each option by calculating the oxidation states of the elements involved.
1. Option A: PbI4
- Let the oxidation state of Pb be x.
- The oxidation state of iodine (I) is −1.
- The equation for the compound is:
x+4(−1)=0
- Simplifying gives:
x−4=0⟹x=+4
- Lead (Pb) in the +4 oxidation state can act as an oxidizing agent. However, I- is a weak reducing agent and cannot reduce Pb from +4 to 0. Therefore, PbI4 is not stable and the compound is not possible.
2. Option B: AlO2
- Let the oxidation state of Al be x.
- The oxidation state of oxygen (O) is −2.
- The equation for the compound is:
x+2(−2)=0
- Simplifying gives:
x−4=0⟹x=+4
- The maximum oxidation state of aluminum (Al) is +3. Therefore, AlO2 is not possible.
3. Option C: NaF2
- Let the oxidation state of Na be x.
- The oxidation state of fluorine (F) is −1.
- The equation for the compound is:
x+2(−1)=0
- Simplifying gives:
x−2=0⟹x=+2
- Sodium (Na) typically has an oxidation state of +1. Therefore, NaF2 is not possible.
4. Option D: PbO2
- Let the oxidation state of Pb be x.
- The oxidation state of oxygen (O) is −2.
- The equation for the compound is:
x+2(−2)=0
- Simplifying gives:
x−4=0⟹x=+4
- Lead (Pb) can exist in the +4 oxidation state, making PbO2 (lead dioxide) a valid compound. This compound is known to be used in batteries as an electrode.
Conclusion:
The only possible compound among the options given is PbO2.
- Let the oxidation state of Pb be x.
- The oxidation state of iodine (I) is −1.
- The equation for the compound is:
x+4(−1)=0
- Simplifying gives:
x−4=0⟹x=+4
- Lead (Pb) in the +4 oxidation state can act as an oxidizing agent. However, I- is a weak reducing agent and cannot reduce Pb from +4 to 0. Therefore, PbI4 is not stable and the compound is not possible.
- Let the oxidation state of Al be x.
- The oxidation state of oxygen (O) is −2.
- The equation for the compound is:
x+2(−2)=0
- Simplifying gives:
x−4=0⟹x=+4
- The maximum oxidation state of aluminum (Al) is +3. Therefore, AlO2 is not possible.
- Let the oxidation state of Na be x.
- The oxidation state of fluorine (F) is −1.
- The equation for the compound is:
x+2(−1)=0
- Simplifying gives:
x−2=0⟹x=+2
- Sodium (Na) typically has an oxidation state of +1. Therefore, NaF2 is not possible.
- Let the oxidation state of Pb be x.
- The oxidation state of oxygen (O) is −2.
- The equation for the compound is:
x+2(−2)=0
- Simplifying gives:
x−4=0⟹x=+4
- Lead (Pb) can exist in the +4 oxidation state, making PbO2 (lead dioxide) a valid compound. This compound is known to be used in batteries as an electrode.
The only possible compound among the options given is PbO2.
Topper's Solved these Questions
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY SEC - 1
12 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PART - III CHEMISTRY SEC - 2
18 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
- TEST SERIESBOOK - RESONANCE ENGLISHCHAPTER - TEST SERIESEXERCISE - CHEMISTRY
50 Videos
Similar Questions
Knowledge Check
Which of the following reaction is possible ?
AC6H5OH+HBr→C6H5Br+H2OB(CH3)3CCl+NaOCH3→(CH3)3COCH3+NaClC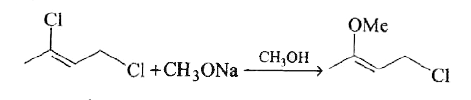 D
D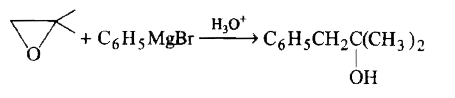
Which of the following reaction is possible ?
AC6H5OH+HBr→C6H5Br+H2OB(CH3)3CCl+NaOCH3→(CH3)3COCH3+NaClC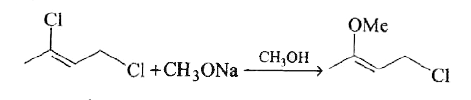 D
D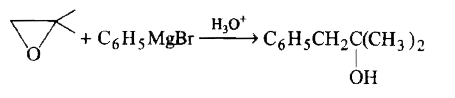
Which of the following reaction is possible ?
AC6H5OH+HBr→C6H5Br+H2OB(CH3)3CCl+NaOCH3→(CH3)3COCH3+NaClC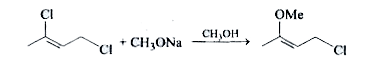 D
D
Which of the following change is possible in aqueous medium -
View SolutionWhich of the following compounds will give a coloured crystalline compound with
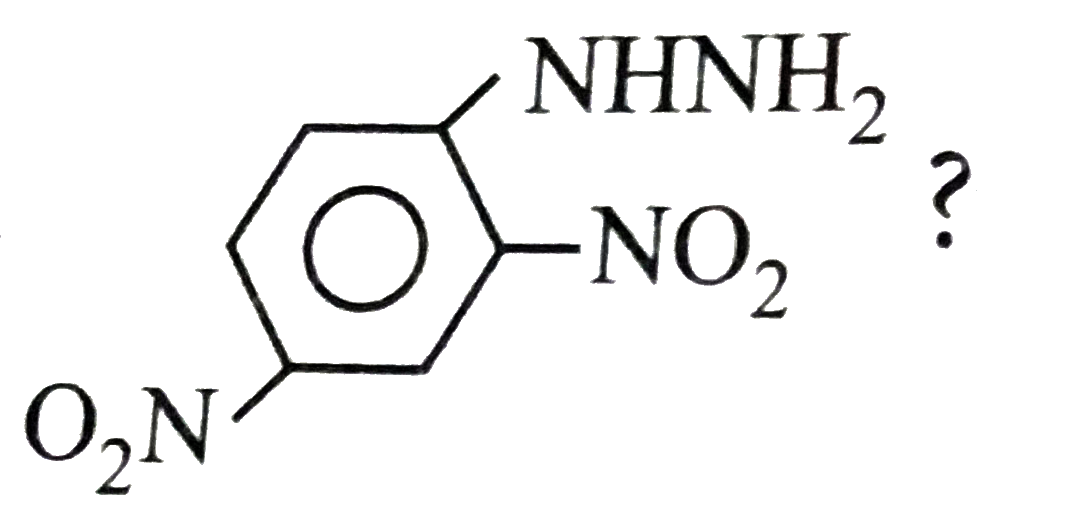 ?
?
View SolutionWhich of the following reactions are possible ?
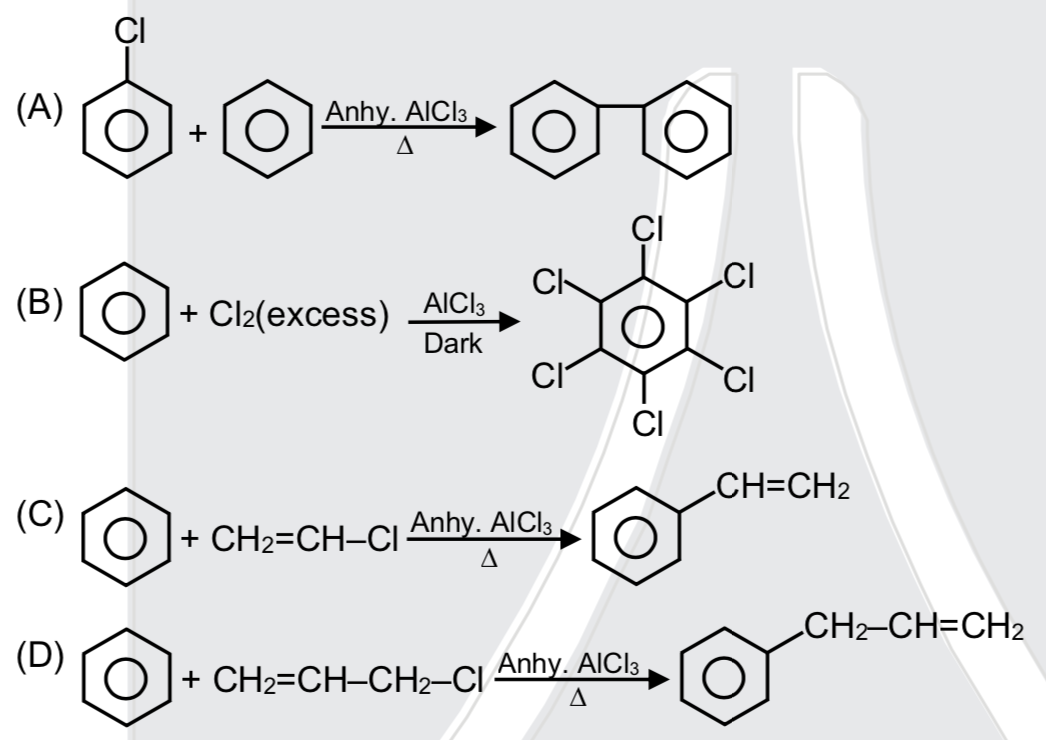
View SolutionWhich of the following reaction is possible at anode ?
View SolutionWhich of the following compound is non-resovable (meso) compounds ?
View Solution

