A Carnot engine takes 3×106cal. of heat from a reservoir at 627∘C, and gives it to a sink at 27∘C. The work done by the engine is
4.2×106J
8.4×106J
16.8×106J
zero
The correct Answer is:D
T1=627+273=900K
Q1=3×106cal
T2=27+273=300K
∴Q1T1=Q2T2
Q2=T2T1×Q1=300900×3×106=1×106cal
Work done =Q1−Q2
=3×106−1×106=2×106
=2×4.2×106J=8.4×106J
Topper's Solved these Questions
Similar Questions
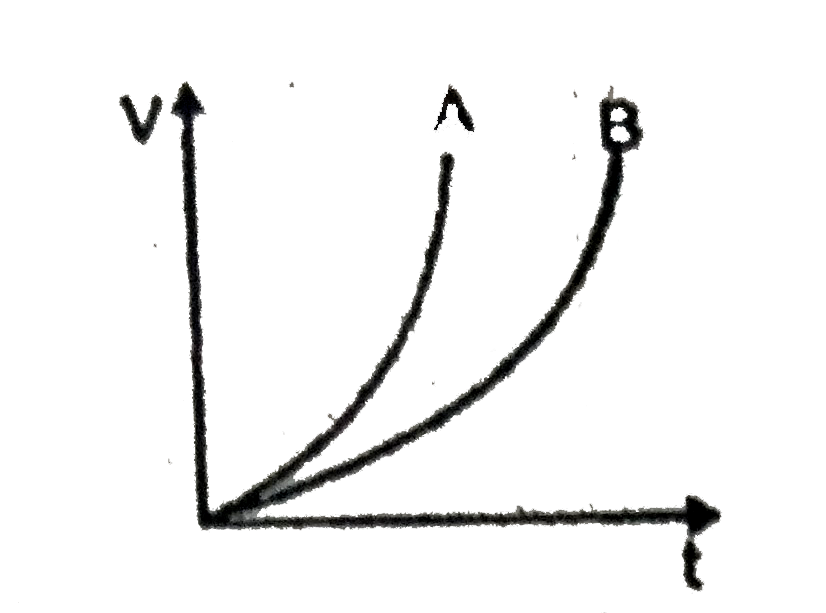
A velocity-time graph of two vehicals A & B starting from rest at the same time is given in the figure. The statements which is correct from graph.
View SolutionA particle performs SHM on x- axis with amplitude A and time period period T .The time taken by the particle to travel a distance A/5 string from rest is
View SolutionA Carnot engine takes 3xx10^6cal . of heat from a reservoir at 62^@C , and gives it to a sink at 27^@C . The work done by the engine is
View SolutionA Carnot engine takes 3×106 cal of heat from a reservoir at 627∘C and gives it to a sink at 27∘C. The work done by the engine is:
View SolutionA carnot engine takes 6000 cal of heat from a reservoir at 627^(@)C and gives it to a sink at 27^(@)C . The work done by the engine is
View SolutionA Carnot engine takes 3 × 106 cal of heat from a reservoir at 627°C, and gives to a sink at 27°C. The work done by the engine is
View SolutionA carnot engine takes in 3000 kcal of heat from a reservoir at 627^@C and gives a part of it to a sink at 27^@C . The work done by the engine is
View SolutionA Carnot engine takes 9000 cal of heat from a reservoir at 627^@ C and gives it to a sink at 27^@C . The work done by the angine is
View SolutionA Carnot engine takes 3000 kcal of heat from a reservoir at 627∘C. The sink is at 27∘C. What is the amount of work done by the engine?
View SolutionA Carnot engine takes 3 xx 10^(6) cals of heat from a reservoir at 627^(@)C and gives it to a sink at 27^(@)C . The work done by the engine is:
View SolutionA carnot engine takes in 3000 kcal of heat from a reservoir at 627°C and gives it to a sink at 27°C. The work done by the engine is
View SolutionA Carnot engine takes 3×106 cal of heat from a reservoir at 627∘C and gives it to a sink 27∘C. The work done by the engine is
View SolutionA Carnot engine takes 3×106 cal of heat from a reservoir at 627∘C and gives it to a sink at 27∘C. The work done by the engine is:
View SolutionA Carnot engine takes 3×106 cal of heat from a reservoir at 627∘C and gives it to sink at 27∘C. What is the work done by. the engine ?
View SolutionA Carnot engine takes 3×106 ca. of heat froma reservoir at 627∘C, and gives it to a sink at 27∘c. The work done by the engine is
View SolutionA Carnot engine takes 3xx10^6cal . of heat from a reservoir at 627^@C , and gives it to a sink at 27^@C . The work done by the engine is
View Solution

