Statement 1: A gas has a unique value of specific heat.
Statement 2: Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
The correct Answer is:D
To analyze the statements provided in the question, we will break down each statement and evaluate its correctness step by step.
Step 1: Evaluate Statement 1
Statement 1: A gas has a unique value of specific heat.
- Analysis:
- Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin).
- For gases, the specific heat can vary depending on the conditions under which the gas is measured. Specifically, there are two common conditions: constant volume (Cv) and constant pressure (Cp).
- Thus, a gas does not have a unique value of specific heat; it can have different values based on the conditions (Cv and Cp) and can also change with temperature.
Conclusion for Statement 1: This statement is false.
Step 2: Evaluate Statement 2
Statement 2: Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
- Analysis:
- The definition provided in Statement 2 is generally correct; specific heat is indeed defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree.
- However, the specific heat can vary depending on the conditions (e.g., whether the process occurs at constant volume or constant pressure) and can also depend on the temperature of the substance.
- Therefore, while the definition is accurate, it does not account for the fact that specific heat can change under different conditions.
Conclusion for Statement 2: This statement is partially true but misleading as it does not emphasize the variability of specific heat under different conditions.
Final Conclusion
- Statement 1: False
- Statement 2: Partially true but misleading
Summary
Both statements have inaccuracies regarding the concept of specific heat. Statement 1 is clearly false, while Statement 2 lacks the necessary context about the variability of specific heat.
---
Statement 1: A gas has a unique value of specific heat.
- Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius (or one Kelvin).
- For gases, the specific heat can vary depending on the conditions under which the gas is measured. Specifically, there are two common conditions: constant volume (Cv) and constant pressure (Cp).
- Thus, a gas does not have a unique value of specific heat; it can have different values based on the conditions (Cv and Cp) and can also change with temperature.
Statement 2: Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
- The definition provided in Statement 2 is generally correct; specific heat is indeed defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree.
- However, the specific heat can vary depending on the conditions (e.g., whether the process occurs at constant volume or constant pressure) and can also depend on the temperature of the substance.
- Therefore, while the definition is accurate, it does not account for the fact that specific heat can change under different conditions.
- Statement 1: False
- Statement 2: Partially true but misleading
Both statements have inaccuracies regarding the concept of specific heat. Statement 1 is clearly false, while Statement 2 lacks the necessary context about the variability of specific heat.
Topper's Solved these Questions
Similar Questions
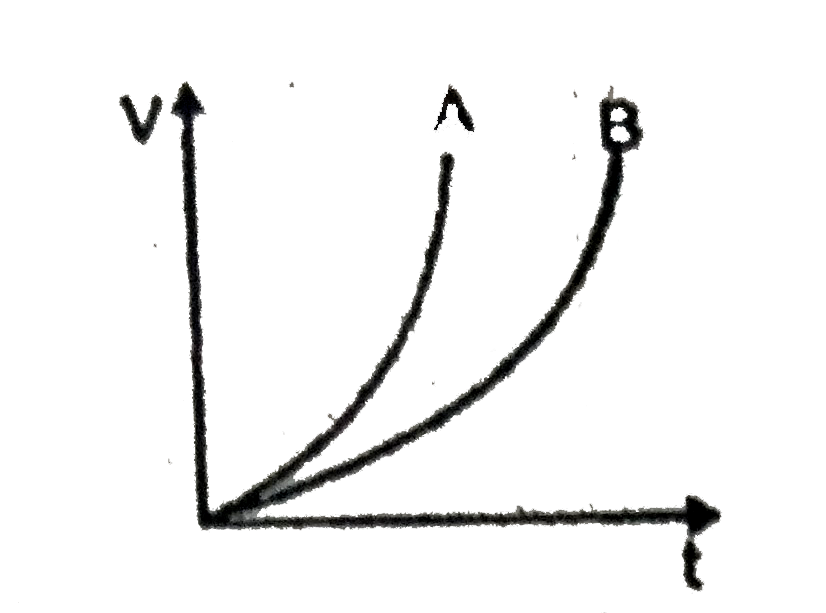
A velocity-time graph of two vehicals A & B starting from rest at the same time is given in the figure. The statements which is correct from graph.
View SolutionA particle performs SHM on x- axis with amplitude A and time period period T .The time taken by the particle to travel a distance A/5 string from rest is
View SolutionAmout of heat required to raise the temperature of unit mass of the substance through unit degree is called……..of the substance.
View SolutionAssertion: Specific heat of a body is always greater than its thermal capacity.
Reason: Thermal capacity is the heat required for raising temperature of unit mass of the body through unit degree
View SolutionHow much heat (in joule) is required to raise the temperature of 205g of water from 21.2∘C to 91.4∘C. Specific heate of water is 4.18Jg−1.∘C−1
Strategy : The specific heat of a substance is the amount of heat required to raise the temperature of 1g of the substance by 1∘C.
Thus
Specific heat
=(Amount of heat in joules)(Mass of substance in grams)(Temperature change in.∘C)
Rearrangement gives.
Amount of heat (q)= Mass of substance (m)× Specific heat
(c)× Temperature change (ΔT)
View SolutionStatement 1: A gas has a unique value of specific heat.
Statement 2: Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
View SolutionStatement - 1: An ideal gas has infinitely many molar specific heats.
Statement-2: Specific heat is amount of heat needed to raise the temperature of 1 mole of gas by 1K.
View SolutionThe quantity of heat required to raise the temperature fo a unit mass of a substance through one degree celsius called ____
View SolutionThe value of heat required to raise the temperature of unit mass of gas through 1 K is
View SolutionThe amount of heat energy required to increase the object temperature by 1∘C is called ______ .
View SolutionSpecific heat is defined as the heat required to raise the temperature of 1 kg substance through 1 K. The unit of specific heat in S.I is
View SolutionAssertion : A gas have a unique value of specific heat. Reason : Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
View SolutionStatement I : A gas have a unique value of specific heat. Statement II : Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
View Solutionप्रक्कथन : गैस की विशिष्ट ऊष्मा का मान अद्वितीय (uniaue) होता है।
कारण : विशिष्ट ऊष्मा, ऊष्मा की वह मात्रा है, जो पदार्थ की इकाई द्रव्यमान का ताप इकाई डिग्री से बढ़ाने के लिए आवश्यक होती है।
View Solution(A): Specific heat is defined as the amount of heat required to raise the temperatrue of unit mass of the substance through unit degree.
(R): A gas has unique value of specific heat
View Solution
