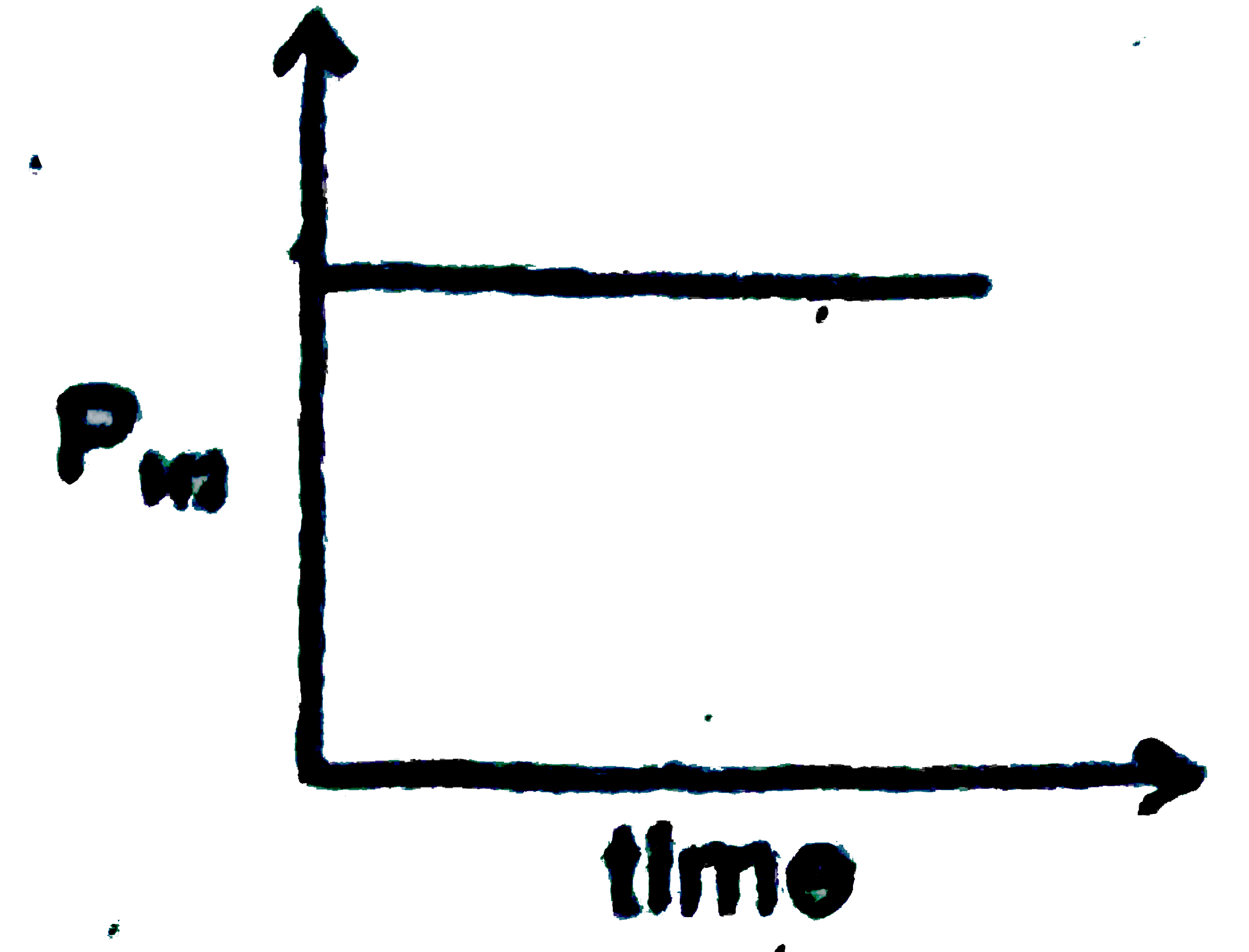
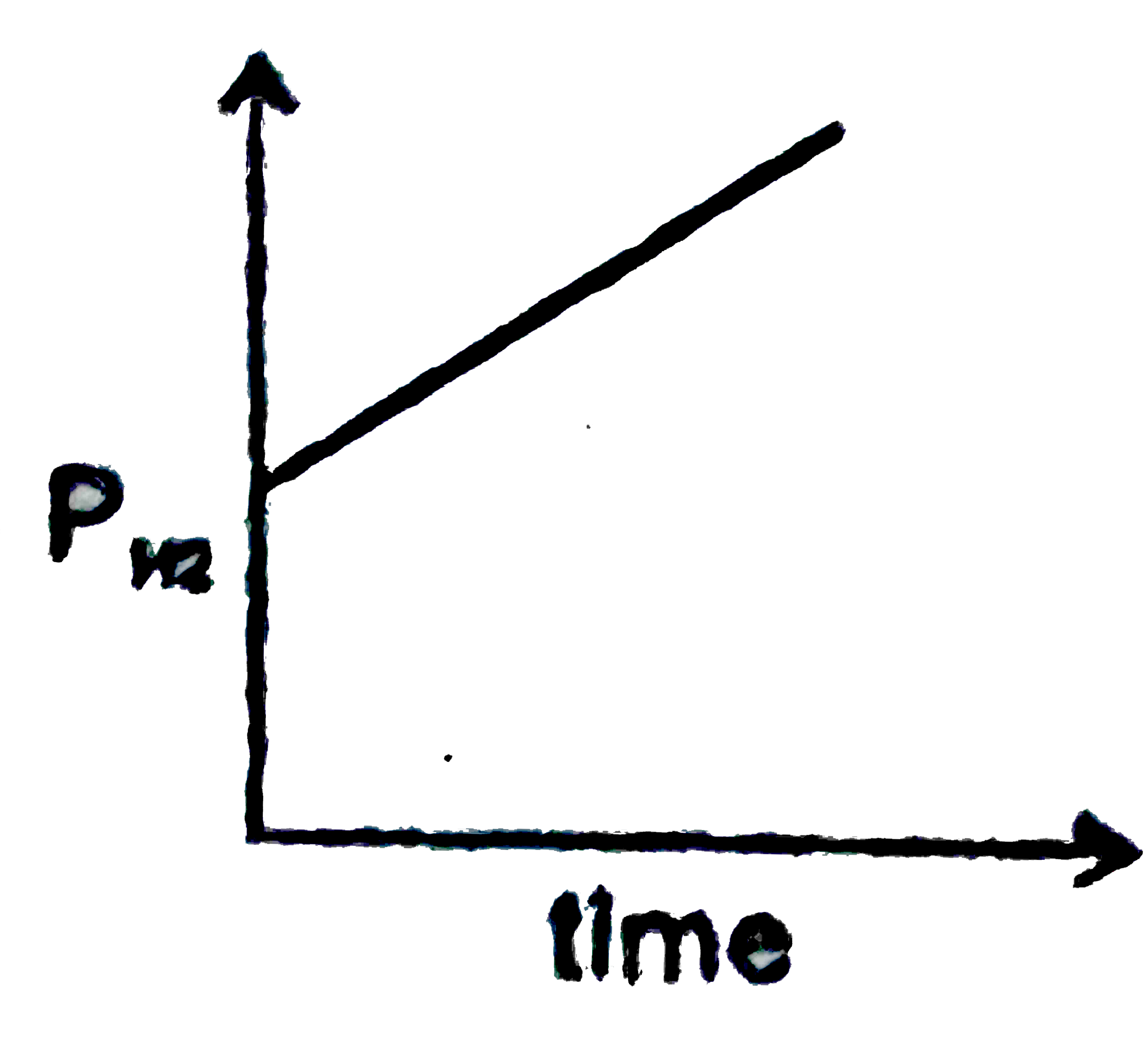
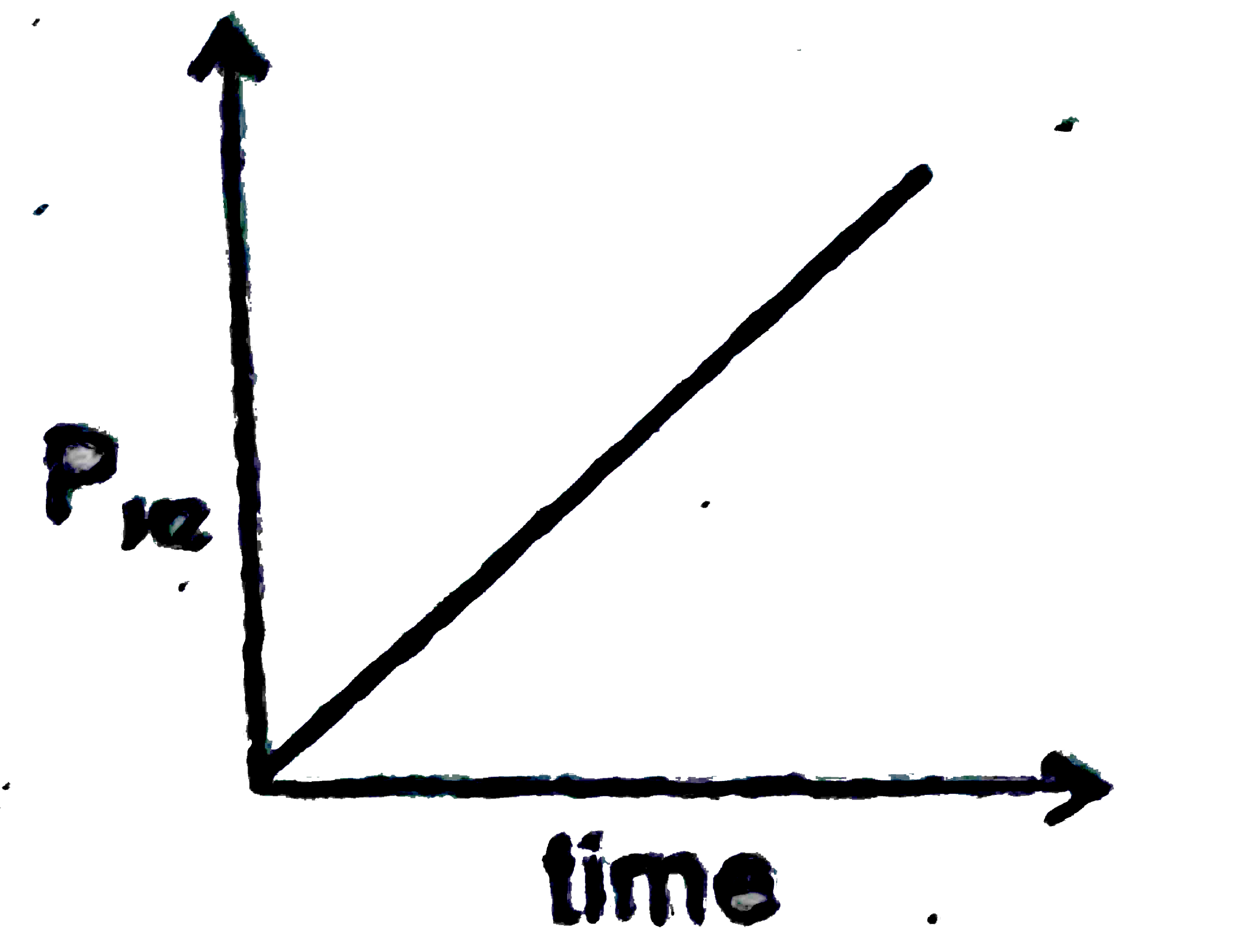
Decomposition of Hl(g) on Gold surface is zero order reaction. Initially, few moles of H2 are present in container then which of the following graph is correct ?
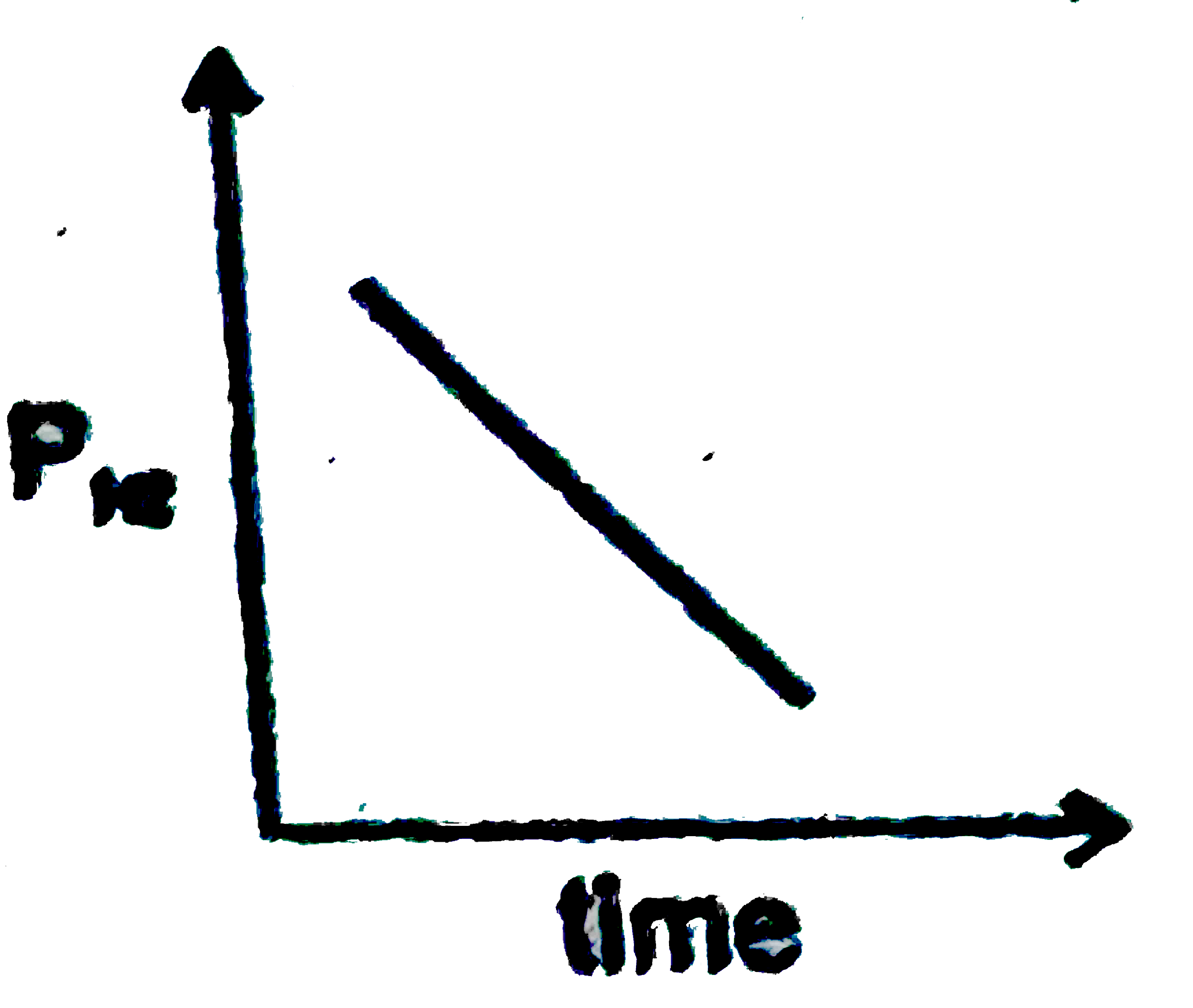
The correct Answer is:2
To solve the question regarding the decomposition of HI (g) on a gold surface, which is a zero-order reaction, we can follow these steps:
1. Understanding Zero-Order Reactions:
- In a zero-order reaction, the rate of reaction is constant and does not depend on the concentration of the reactants. The rate law can be expressed as:
Rate=k
- The integrated rate law for a zero-order reaction is given by:
(A)=(A)0−kt
where (A) is the concentration at time t, (A)0 is the initial concentration, and k is the rate constant.
2. Graphical Representation:
- For a zero-order reaction, if we plot the concentration of the reactant (in this case, HI) against time, the graph will be a straight line with a negative slope. This indicates that the concentration decreases linearly over time.
3. Initial Concentration of H2:
- The problem states that initially, there are a few moles of H2 present. This means that at t=0, the concentration of H2 is not zero.
4. Analyzing the Options:
- Since the graph of a zero-order reaction will show a linear decrease in concentration, we need to identify which graph correctly represents this behavior while also accounting for the initial concentration of H2.
- The options presented likely include graphs that either start from zero or have a negative slope. Since we have an initial concentration of H2, the graph must start from a positive value and decrease over time.
5. Conclusion:
- The correct graph will be the one that starts from a positive concentration of H2 and shows a linear decrease over time. Therefore, the correct option is B, which represents the behavior of a zero-order reaction with an initial concentration of H2.
Final Answer:
The correct graph is option B.
- In a zero-order reaction, the rate of reaction is constant and does not depend on the concentration of the reactants. The rate law can be expressed as:
Rate=k
- The integrated rate law for a zero-order reaction is given by:
(A)=(A)0−kt
where (A) is the concentration at time t, (A)0 is the initial concentration, and k is the rate constant.
- For a zero-order reaction, if we plot the concentration of the reactant (in this case, HI) against time, the graph will be a straight line with a negative slope. This indicates that the concentration decreases linearly over time.
- The problem states that initially, there are a few moles of H2 present. This means that at t=0, the concentration of H2 is not zero.
- Since the graph of a zero-order reaction will show a linear decrease in concentration, we need to identify which graph correctly represents this behavior while also accounting for the initial concentration of H2.
- The options presented likely include graphs that either start from zero or have a negative slope. Since we have an initial concentration of H2, the graph must start from a positive value and decrease over time.
- The correct graph will be the one that starts from a positive concentration of H2 and shows a linear decrease over time. Therefore, the correct option is B, which represents the behavior of a zero-order reaction with an initial concentration of H2.
The correct graph is option B.
Topper's Solved these Questions
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Book:RESONANCE ENGLISHChapter:CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Exercise:Part -IV
Explore 21 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:RESONANCE ENGLISHChapter:CHEMISTRY IN EVERYDAY LIFEExercise:ORGANIC CHEMISTRY(Chemistry in every day life)
Explore 31 Videos
Similar Questions
Knowledge Check
Assertion : The decomposition of gaseous ammonia on a hot platinum surface is a zero order reaction at high pressure.
For a zero order reaction the rate of reaction is independent of initial concentration.AIf both assertion and reason are true and reason is the correct explanation of assertion.BIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assertion is true but reason is false.DIf both assertion and reason are false.
Which of the following is correct graph for the reaction?
View SolutionIn which of the following reactions H2(g) is produced?
View SolutionWhich of the following graph is correct for a zero order reaction ?
View SolutionWhich of the following graph is correct for a first order reaction ?
View SolutionIf reaction A+B⇔C+D, taken place in 5 liter close vessel, the rate constant of forward reaction is nine times of rate of backward reaction.
If initially one mole of each reactant present in the container, then find the correct option//is.
View SolutionIn a 20 litre vessel at 300 K initially, 1 mole of CO,H2O,CO2 are present, then for the equilibrium of CO+H2O⇔CO2+H2 which of the following is true?
View SolutionFor the reaction
N2(g)+3H2(g)→2NH2(g)
Which of the following is correct?
View Solution
