In a reaction A→ produce, when from 8.0×10−2 M of A, half life is found to be 120 minute. For the initial concentraton 4.0×10−2M, the half life of the reaction become 240 minute. The order of the reaction is :
zero
one
two
0.5
The correct Answer is:3
(t1/2)1(t1/2)2=(a2a1)n−1,120240=(4×10−28×10−2)n−1,n=2
Topper's Solved these Questions
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Book:RESONANCE ENGLISHChapter:CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID Exercise:Part -IV
Explore 21 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:RESONANCE ENGLISHChapter:CHEMISTRY IN EVERYDAY LIFEExercise:ORGANIC CHEMISTRY(Chemistry in every day life)
Explore 31 Videos
Similar Questions
The product of the reaction
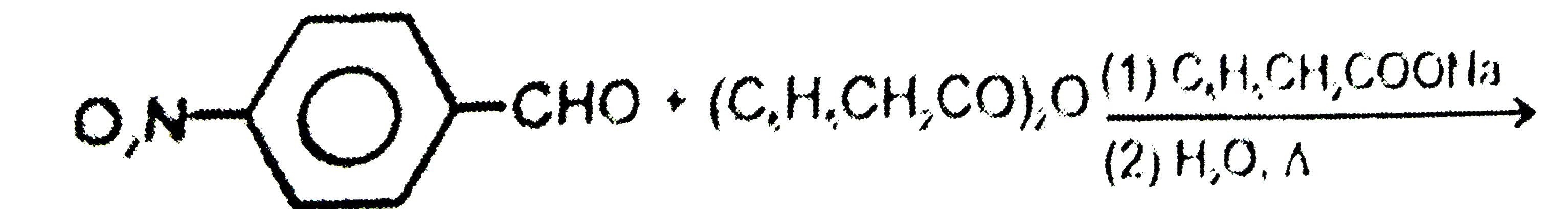
View SolutionWhich is the correct statement about birth control pills?
View SolutionIn a reaction A→ products , when start is made from 8.0×10−2M of A, half life is found to be 120 minute. For the initial concentration 4.0×10−2M, the half life of the reaction becomes 240 minute. The order of the reaction is :
View SolutionThe half period for the decomposition of a compound is 20 minutes. If the initial concentration is made twice, the half life period becomes 10 minutes. Calculate the order of reaction.
View Solutionएक प्रथम कोटि की अभिक्रिया का अर्ध-आयु काल 10 मिनट है यदि अभिक्रिया प्रारंभिक सान्द्रता12M के साथ सुरु की जाती है तो 20 मिनट दर होगी
View SolutionIn a reaction A→ products, when start is made from 8.0×10−2M of A, half-life is found to be 120minute. For the initial concentration 4.0×10−2M, the half-life of the reaction becomes 240 minutes. The order of the reaction is :
View SolutionThe half-life period of a first order reaction is 60 minutes. What percentage will be left after 240 minutes ?
View SolutionThe half life period of a substance is 50 minutes at a certain initial concentration. When the concentration is reduced to one half of its initial concentration, the half life period is found to be 25 minutes. Calculate the order of reaction.
View SolutionAt particular concentration, the half life of the reaction is 100 minutes. When the concentration of reactant becomes double half life becomes 25 minutes, then what will be the order of the reaction ?
View SolutionAt a given initial concentration of reactant , the half-life of a reaction is found to be 50min . If the initial concentration is quadrupled , then half-life of the reaction becomes 25min . The order of the reaction is -
View SolutionThe half life period of a substance is 50 minutes at a certain initial concentration. When the concentration is reduced to one half of the initial value, the half-life period is 25 minutes. Calculate the order of the reaction.
View SolutionAt particular concentration , the half life of the reaction is 100 minutes. When the concentration of the reactant become double half life becomes , 25 minutes , then what will be the order of the reaction ?
View SolutionReaction P→ Q The half-life of the reaction and concentration of P is given in graph.
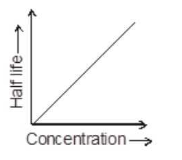
Concentration Concentration of P decreases from 1 M to 0.25 M in 100 minute, the initial rate of reaction (in M/minute)
View Solutionप्रथम कोटि अभिक्रिया के लिए अर्द्ध आयुकाल 60 मिनिट है 240 मिनिट बाद कितना प्रतिशत शेष बचेगा ।
View SolutionHalf life periods for a reaction at initial concentration of 0.1 M and 0.01 M are 5 and 50 minutes respectively. Then the order of reaction is
View SolutionThe half-life period of a 1st order reaction is 60 minutes. What percentage will be left over after 240 minutes ?
View Solution
