For the reaction,
2NO(g)+2H2(g)→N2(g)+2H2O(g)
The rate expression can be written in the following ways:
d[N2]dt=k1[NO][H2],d[H2O]dt=k2[NO][H2]
−d[NO]dt=k3[NO][H2],−d[H2]dt=k4[NO][H2]
The relationship between k1,k2,k3,k4 is
k1=k2=k3=k4
k1=2k2=k3=k4
2k1=k2=k3=k4
2k1=2k2=k3=2k4
The correct Answer is:2
To find the relationship between the rate constants k1,k2,k3, and k4 for the reaction
2NO(g)+2H2(g)→N2(g)+2H2O(g),
we will analyze the given rate expressions step by step.
Step 1: Write the rate expressions
The rate expressions provided are:
1. d(N2)dt=k1(NO)(H2)
2. d(H2O)dt=k2(NO)(H2)
3. −d(NO)dt=k3(NO)(H2)
4. −d(H2)dt=k4(NO)(H2)
Step 2: Relate the rates of formation and consumption
From stoichiometry, we know that the rates of formation and consumption are related by the coefficients in the balanced equation. Therefore, we can express the rates in terms of a common rate R:
R=12(−d(NO)dt)=12(−d(H2)dt)=d(N2)dt=12d(H2O)dt
Step 3: Establish relationships between k1,k2,k3, and k4
1. From the first and third expressions:
R=12k3(NO)(H2)andR=k1(NO)(H2)
Equating these gives:
k1(NO)(H2)=12k3(NO)(H2)
Canceling (NO)(H2) (assuming they are not zero):
k3=2k1
2. From the second and fourth expressions:
R=12k4(NO)(H2)andR=12k2(NO)(H2)
Equating these gives:
12k4(NO)(H2)=12k2(NO)(H2)
Canceling (NO)(H2):
k4=k2
3. From the first and second expressions:
R=k1(NO)(H2)andR=12k2(NO)(H2)
Equating these gives:
k1(NO)(H2)=12k2(NO)(H2)
Canceling (NO)(H2):
k2=2k1
Step 4: Summarize the relationships
From the above steps, we have established the following relationships:
- k3=2k1
- k4=k2
- k2=2k1
Thus, we can conclude:
k3=k4=2k1
Final Relationship
Putting it all together, we find:
k2=2k1,k3=2k1,k4=2k1
So, the final relationship can be summarized as:
k2=k3=k4=2k1
The rate expressions provided are:
2. d(H2O)dt=k2(NO)(H2)
3. −d(NO)dt=k3(NO)(H2)
4. −d(H2)dt=k4(NO)(H2)
From stoichiometry, we know that the rates of formation and consumption are related by the coefficients in the balanced equation. Therefore, we can express the rates in terms of a common rate R:
1. From the first and third expressions:
R=12k3(NO)(H2)andR=k1(NO)(H2)
Equating these gives:
k1(NO)(H2)=12k3(NO)(H2)
Canceling (NO)(H2) (assuming they are not zero):
k3=2k1
R=12k4(NO)(H2)andR=12k2(NO)(H2)
Equating these gives:
12k4(NO)(H2)=12k2(NO)(H2)
Canceling (NO)(H2):
k4=k2
R=k1(NO)(H2)andR=12k2(NO)(H2)
Equating these gives:
k1(NO)(H2)=12k2(NO)(H2)
Canceling (NO)(H2):
k2=2k1
From the above steps, we have established the following relationships:
- k3=2k1
- k4=k2
- k2=2k1
k3=k4=2k1
Putting it all together, we find:
k2=2k1,k3=2k1,k4=2k1
So, the final relationship can be summarized as:
k2=k3=k4=2k1
Topper's Solved these Questions
- CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID BOOK - RESONANCE ENGLISHCHAPTER - CARBONYL COMPOUNDS (ALDEHYDES & KETONES ) & CARBOXYLIC ACID EXERCISE - Part -IV
21 Videos
- CHEMISTRY IN EVERYDAY LIFEBOOK - RESONANCE ENGLISHCHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - ORGANIC CHEMISTRY(Chemistry in every day life)
31 Videos
Similar Questions
The product of the reaction
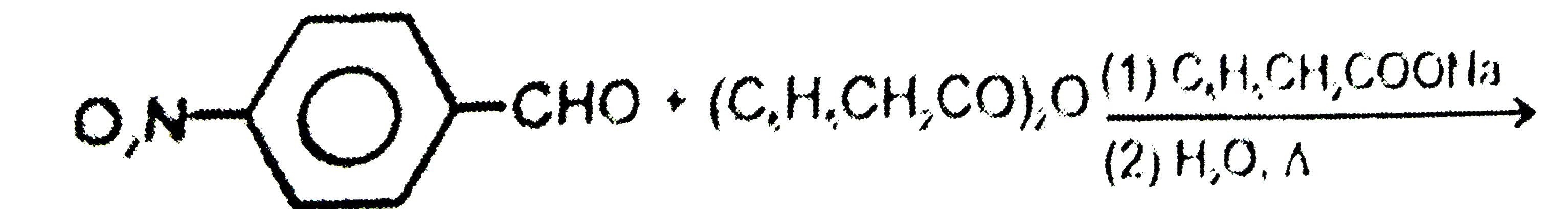
View SolutionWhich is the correct statement about birth control pills?
View SolutionThe relation between K_(p) and K_(c) is K_(p)=K_(c)(RT)^(Deltan) unit of K_(p)=(atm)^(Deltan) , unit of K_(c)=(mol L^(-1))^(Deltan) Consider the following reactions: i. CO(g)+H_(2)O(g) hArr CO_(2)(g)+H_(2)(g), K_(1) ii. CH_(4)(g)+H_(2)O(g) hArr CO(g)+3H_(2)(g), K_(2) iii. CH_(4)(g)+2H_(2)O(g) hArr CO_(2)(g)+4H_(2)(g), K_(3) Which of the following is correct?
View SolutionFor the reaction,
2NO(g)+2H2(g)→N2(g)+2H2O(g)
The rate expression can be written in the following ways:
d[N2]dt=k1[NO][H2],d[H2O]dt=k2[NO][H2]
−d[NO]dt=k3[NO][H2],−d[H2]dt=k4[NO][H2]
The relationship between k1,k2,k3,k4 is
View SolutionFor the following reactions (1) , (2) and (3) equilibrium constants are given (1) CO_(2)(g) +H_(2)O(g) hArr CO_(2)(g) +H_(2)(g), K_(1) (2) CH_(4)(g) +H_(2)O(g) hArr CO(g)+3H_(2)(g),K_(2) (3) CH_(4)(g)+2H_(2)O(g) hArr CO_(2)(g)+4H_(2)(g), K_(3) Which of the following relation is correct ?
View SolutionThe equilibrium constants for the following reactions
N2(g)+3H2(g)⇔2NH3(g)N2(g)+O2(g)⇔2NO(g)
and H2(g)+1/2O2(g)⇔H2O(Ig) are K1,K2 and K3 respectively.
The equilibrium constant (K) for the reaction
2NH3(g)+21/2)O2(g)⇔2NO(g)+3H2O(I) is
View Solution(i) N2(g)+O2(g)⇔2NO(g),K1
(ii) (12)N2(g)+(12)O2(g)⇔NO(g),K2
(iii) 2NO(g)⇔N2(g)+O2(g),K3
(iv) No(g)⇔(12)N2(g)+(12)O2(g),K4
Correct relation between K1,K2,K3andK4 is//are:
View SolutionConsider the reactions CO_((g))+H_(2)O_((g)to).^(larr)CO_(2)(g)+H_(2(g)) to K_(1) (ii) CH_(4(g))+H_(2)O_((g)to).^(larr)CO_((g))+3H_(2(g)) - K_(2) (iii) CO_(4(g))+2H_(2)O_((g)to).^(larr)CO_(2(g)) to K_(3) Give the relation between K_(1), K_(2) " and " K_(3). [K_(1), K_(2) " and " K_(3) are the equilibrium constants of the respective reactions.
View SolutionFor the reaction 2H_(2)(g)+2NO(g) rarr N_(2)(g)+2H_(2)O(g) the observed rate expression is, rate =k_(f)[NO]^(2)[H_(2)]. The rate experssion for the reverse reaction is :
View SolutionNitric oxide reacts withH2 according to the reaction
2NO(g)+2H2(g)→N2(g)+2H2O(g)
What is the relationship among
d[NO]dt=d[H2]dt=d[N2]dt and d[H2O]dt?
View SolutionFor the reaction, 2NO(g)+2H_(2)(g)rarrN_(2)(g)+2H_(2)O(g) the rate expression can be written in the following ways : {(d[N_(2)])/(dt)}=k_(1)[NO][H_(2)], {(d(H_(2)O))/(dt)}=k[NO][H_(2)], {(d[NO])/(dt)}=k_(1)'[NO][H_(2)], {(d[H_(2)])/(dt)}=k_(1)''[NO][H_(2)]. The relationship between k,k_(1),k_(1)' and k_(1)''
View SolutionWrite the equations for the conversion of Kcd to Kp for each of the following reaction.
CO(g)+H2O(g)⇔CO2(g)+H2(g)
View SolutionFor the reaction, 2NH3→N2+3H2
if −d[NH3]dt=k1[NH3],
d[N2]dt=k2[NH3],d[H2]dt=k3[NH3]
then the relation between k1,k2 and k3 is
View SolutionFor the reaction mechanism of the reaction 2NO(g)+2H2(g)→N2(g)+2H2O(g)
(Step I,2NO,k1⇐⇒,N2O2,,,Keq(fast)),(Step II,N2O2+H2,k2−−→,N2O+H2O,,(slow)),(Step III,N2O+H2,k3−−→r,N2+H2O,,(fast))
Expression of rate of reaction is
(Take Keq×k2=k')
View SolutionFor the following three reactions I, II and III, equilibrium constants are given : I. H_(2)(g) + 1/2O_(2)(g) rarr H_(2)O ....K_(1) II. C_(2)H_(4)(g) + 3O_(2)(g) rarr 2CO_(2)(g) + 2H_(2)O(g) ....K_(2) III. C_(2)H_(6)(g) + 7/2O_(2)(g) rarr 2CO_(2)(g) + 3H_(2)O(g) ...K_(3) IV. C_(2)H_(4) + H_(2) rarr C_(2)H_(6) ...K_(4) Which of the following relations is correct?
View SolutionFor the reaction 2H2+2NO→N2+2H2O, the following mechanism has been suggested :
2NO?N2O2 equilibrium constant K1 (fast)
N2O2+H2k2−−→N2O+H2O (slow)
N2O+H2k3−−→N2+H2O (fast)
Establish the rate law for given reaction.
View Solution

