For the decomposition of HI the following logarithmic plot is
shown :[R=1.98cal/mol−K]

The activation energy of the reaction is about
45600cal
13500cal
24600cal
32300cal
The correct Answer is:1
logk=−Ea2.303R1T+constant=−Ea2.303R×10−3×103T+constant
Thus slope of graph will be −Ea×10−32.303R=−40.4
⇒ Ea=2.303×1.98×104=45600cal
Topper's Solved these Questions
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - Pt-05
30 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PT-06
28 Videos
- TEST PAPERSBOOK - RESONANCE ENGLISHCHAPTER - TEST PAPERSEXERCISE - PT-03
27 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
- TEST SERIESBOOK - RESONANCE ENGLISHCHAPTER - TEST SERIESEXERCISE - CHEMISTRY
50 Videos
Similar Questions
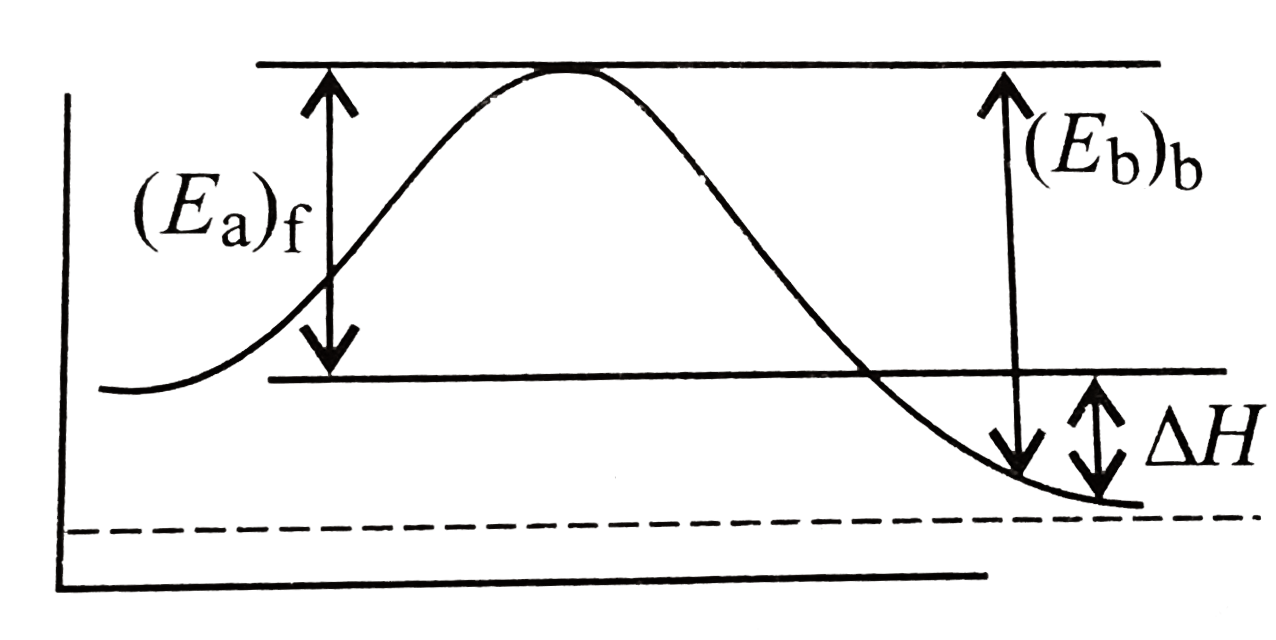
In an exothermic reaction X→Y, the activation energy is 100kJmol−1 of X. The enthalphy of the reaction is −140kJmol−1. The activation energy of the reverse reaction Y→X is
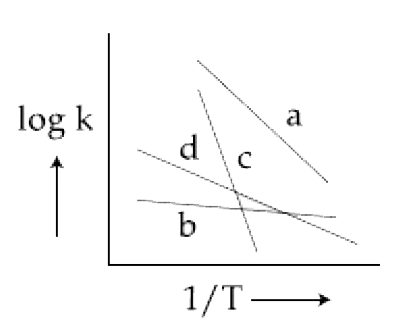
View SolutionConsider the following plots of rate constant versus 1T for four different reactions. Which of the following orders is correct for the activation energies of these reactions ?
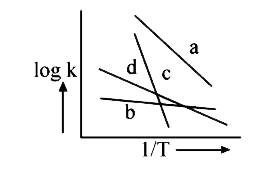
View SolutionConsider the following plots of rate constant versus 1T for four different reactions. Which of the following orders is correct for the activation energies of these reactions?
View SolutionThe activation energy for the forward reaction X→Y is 60kJmol−1 and ΔH is −20kJmol−1. The activation energy for the reverse reaction is
View SolutionThe activation energy of exothermic reaction A→B is 80 kJ mol−1 . The heat of reaction is 200kJmol−1. The activation energy for the reactionB→a ( in kJ/mol) will be :
View SolutionThe rate constant for a first order reaction becomes six times when the temperature is raised from 350 K to 400 K. Calculate the activation energy for the reaction
[R=8.314JK−1mol−1]
View SolutionIn the decomposition of H2O2 at 300 K, the energy of activation was found to be 18kcal/mol white it decreases to 6kcal/mol when the decomposition was carried out in the presence of a catalyst at 300 K. The number of times the catalysed reaction faster than uncatalysed one is (Give you answer by multiplying 107)
View SolutionIf a reaction A+B→C is exothermic to the extent of 30KJmol−1, the the forward reaction has an activation energy , 70KJmol−1, the activation energy for the reverse reaction is
View SolutionA colliison between reactant molecules must occur with a certain minimum energy before it is effective in yielding Product molecules. This minimum energy is called activation energy Ea Large the value of activation energy, smaller the value of rate constant k. Larger is the value of activation energy, greater is the effect of temperature rise on rate constant k.
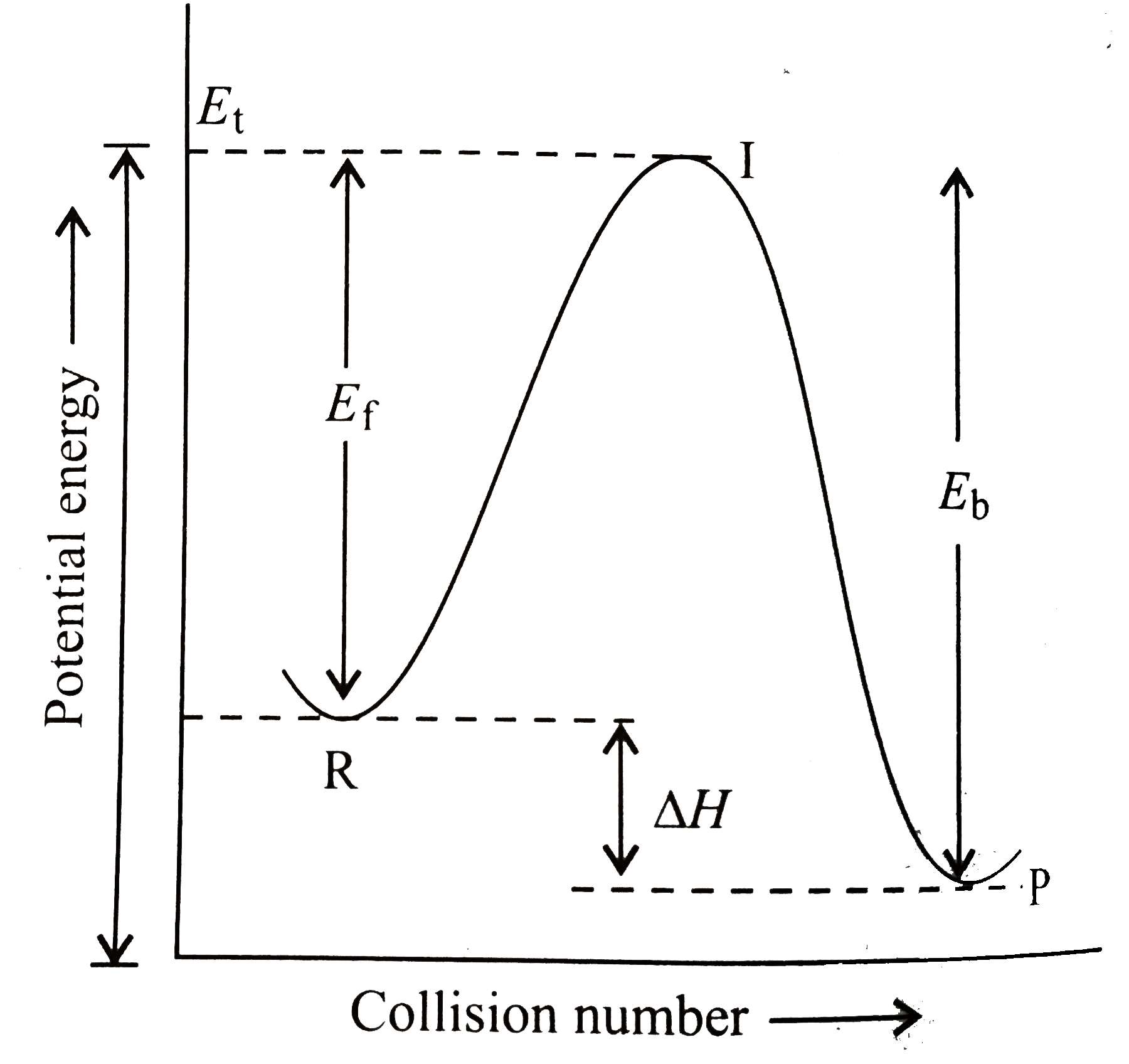
Ef= Activation energy of forward reaction
Eb= Activation energy of backward reaction
ΔH=Ef−Eb
Ef= threshold energy
If a reaction A+B→C is exothermic to the extent 30kJmol−1 and the forward reaction has an activation energy of 249kJmol−1 the activation energy for reverse reaction in kJmol−1 is
View SolutionThe activation energy of a non–catalysed reaction at 37∘C is 200 kcal/mol and the activation energy of the same reaction when catalysed decreases to only 60.0 kcal/mol. Calculate the ratio of rate constants of the two reactions.
View Solution

