What is the time required for 75 percent completion of a first-order reaction?
0.199:1
0.420:1
0.273:1
0.311:1
The correct Answer is:4
Time taken for 25% completion.
C1C0θ−k1t. ⇒34=e−k1t1
as n(3/4)=−k1b1sot1ln(43)
time taken for 75% completion t2=2.ln2k2
so required ration t1t2=ln(4/3)k2k1.2ln2=32×(ln4−ln3)ln4=0.311:1
Topper's Solved these Questions
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:Pt-05
Explore 30 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:PT-06
Explore 28 Videos
- TEST PAPERSBook:RESONANCE ENGLISHChapter:TEST PAPERSExercise:PT-03
Explore 27 Videos
- SURFACE CHEMISTRYBook:RESONANCE ENGLISHChapter:SURFACE CHEMISTRYExercise:Section - 5
Explore 1 Video
- TEST SERIESBook:RESONANCE ENGLISHChapter:TEST SERIESExercise:CHEMISTRY
Explore 50 Videos
Similar Questions
The catalyst used in the manufacture of sulphuric acid by contact process is:
View SolutionWhich of the following compoud on hydrolysis gives acetylene ?
View SolutionRelation between Neopentane and n− pentane is ?
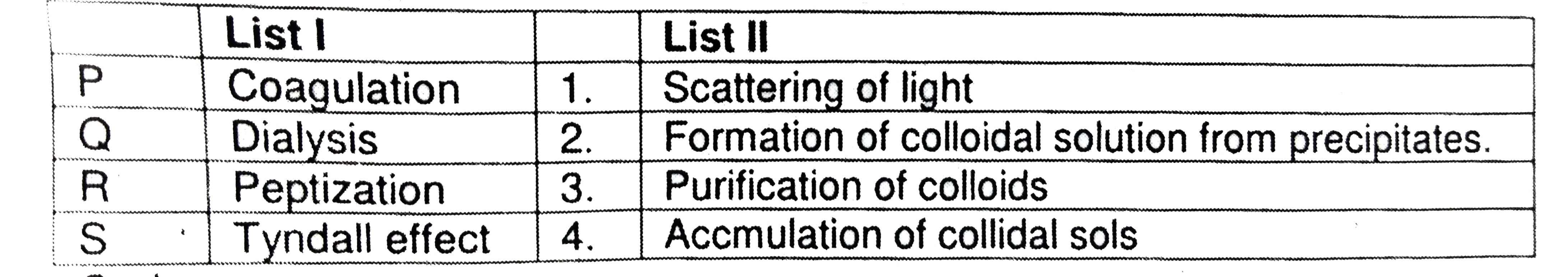
View SolutionMatch list I with list II and select the correct answer :
View Solutionwhich of the following plots will obtained for a conductometric titration of strong acid against a weak base?
View Solution75 % of first order reaction is complete in 30 minutes. What is the time required for 93.75 % of the reaction (in minutes) ?
View SolutionFor the first order reaction at 27^(@)C , the ratio of time required for 75 % completion to 25 % completion of reaction is
View SolutionThe time required for 100 percent completion of a zero order reaction is:
View SolutionThe time required for a first order reaction to complete 90% is 't'. What is the time required to complete 99% of the same reaction?
View SolutionA first order reaction requires 30 minutes for 50 % completion . The time required to complete the reaction by 75 % will be __.
View SolutionTime required for 100 percent completion of a zero order reaction is
View SolutionFor a first order reaction, show that the time required for 99% completion of a first order reaction is twice the time required for the completion of 90% .
View SolutionThe time required for a first order reaction to complete 90% is T. What is the time required to complete 99% of the same reaction?
View Solution75 % of first order reaction is complete in 30 minutes. What is the time required for 93.75 % of the reaction (in minutes) ?
View SolutionThe time required for 100% completion of a zero order reaction is
View SolutionTime required for 50% completion of the first order reaction in one hr. What is the time required for 99% completion of the same reaction ?
View Solution
