In the Lassaigne's test , one of the organic compound gave red colour with FeCl3.Ccompound can be:
Na2S
NH2CSNH2
C6H6Cl
NaCl
The correct Answer is:B
In the Lassaigne's test NH4CSNH2 gave red colour with FeCl3.
Topper's Solved these Questions
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-I)
10 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-II)
8 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise-1 (Part-I)
23 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
23 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
Similar Questions
How many products (structure isomers only) are formed by monochlorination of given compound ?
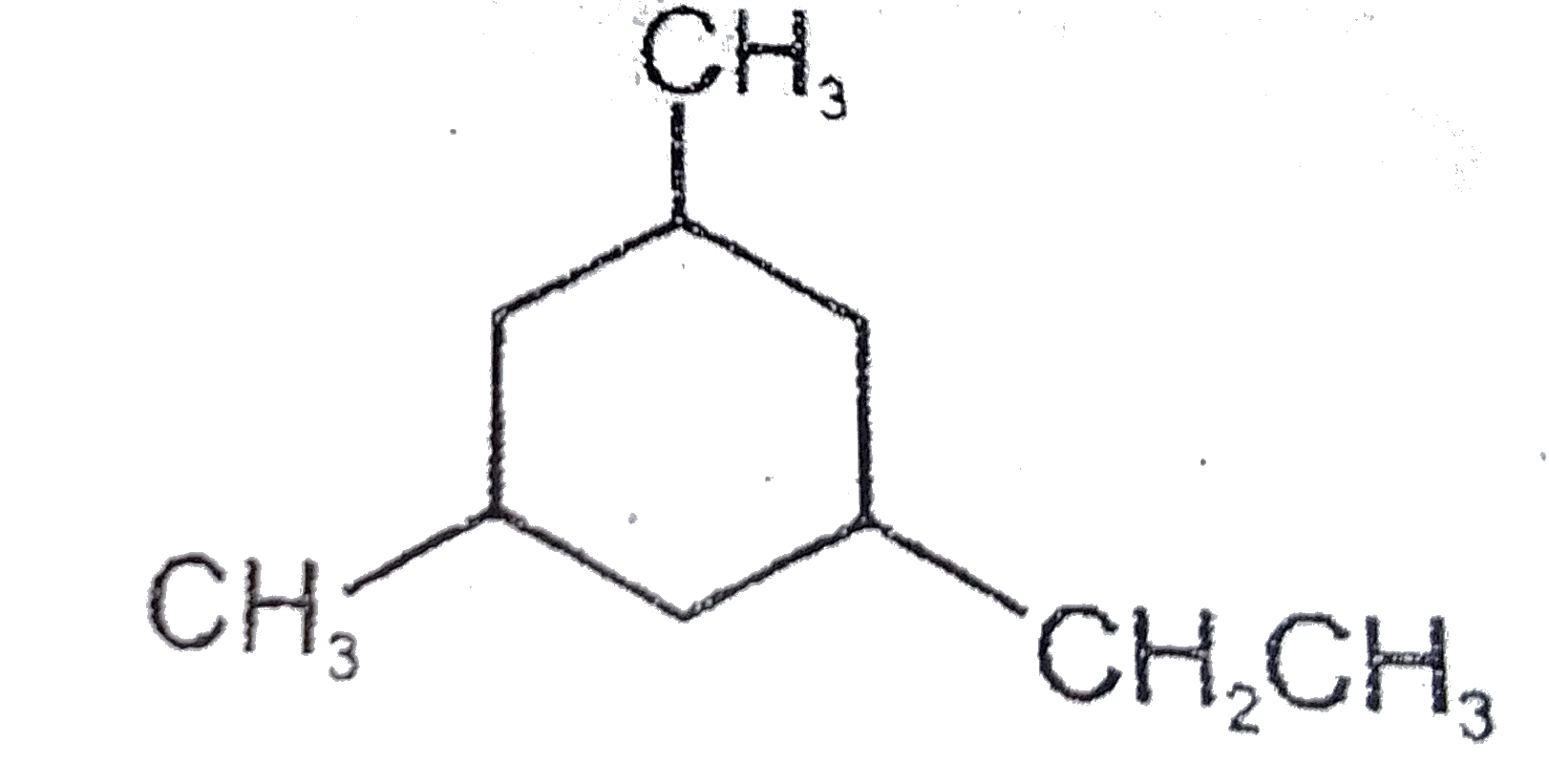
View SolutionHow many isomeric alkynes on catalytic hydrogenation gives 3-Ethyl-4-methylheptane ?
View SolutionCalculate the DU of following compounds :
(i) C6H6ClBrO , (ii) C5H9N
View Solution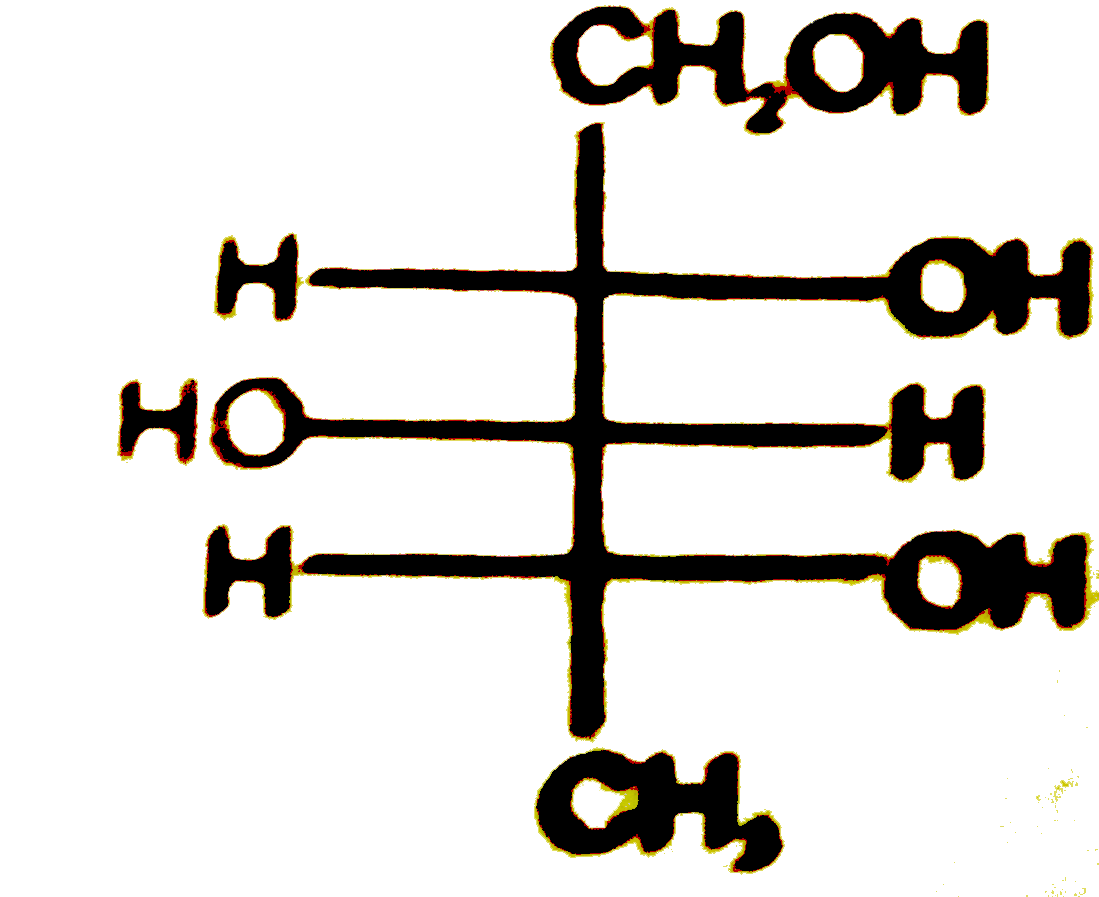 is a Fischer projection of one of_______ stereoisomers ?
is a Fischer projection of one of_______ stereoisomers ?
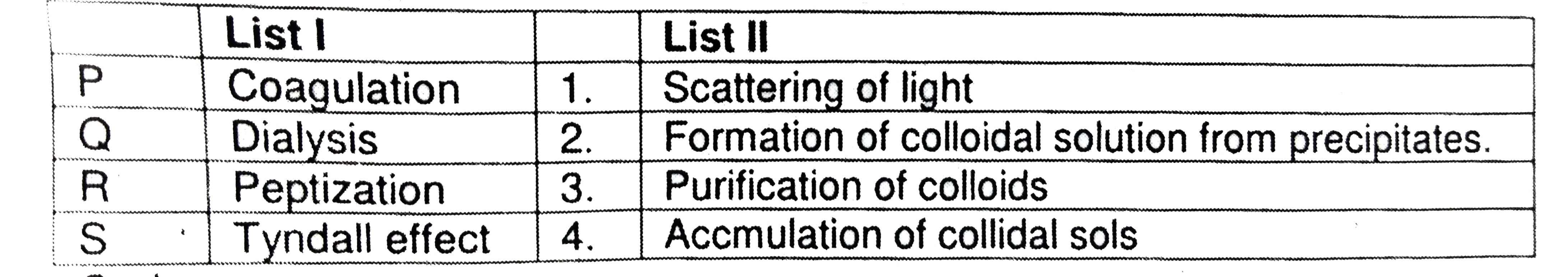
View SolutionMatch list I with list II and select the correct answer :
View SolutionIn Lassaigne's test ,the sodium extract of an organic compound containing both nitrogen and sulpher on treatment with FeCl_(3) produces a blood red colouration due to the formation of
View SolutionIn the Lassaigne's test , one of the organic compound gave red colour with FeCl_(3) .Ccompound can be:
View SolutionWhich of the following organic compound will give red colour in Lassaigne's test?
View SolutionSelect the organic compounds, which will give red colour in Lassaigne's test?
View SolutionIn the Lassaigne's test, one of the organic compounds gave red colour with FeCl3. Compound can be:
View SolutionIn the Lassaigne's test, one of the organic compound X gives blood red colour with FeCl3. Compound X, when fused with sodium metal forms compound Y. Molecular mass of compound Y is
View SolutionThe blood red colour obtained in the detection of nitrogen and sulphur together in an organic compound in Lassaigne's test is due to
View SolutionIn the lassaigne's test , one of the organic compounds gave red colour with FeCl3. Compound can be :
View SolutionIn the Lassaigne's test , one of the organic compound X gives blood red colour with FeCl_(3) . Compound X, when fused with sodium metal forms compound Y. molecular mass of compound Y is
View SolutionSometimes a red colour is not produced in the Lassaigne's test even if both nitrogen and sulphur are present in the organic compound. Explain.
View SolutionIn the Lassaigne's test for the detection of nitrogen in the organic compound, the appearance of blue coloured compound is
View Solution

