Which of the following would produce effervescence with sodium bicarbonate ?
All of these
The correct Answer is:D
To determine which of the given compounds would produce effervescence with sodium bicarbonate (NaHCO₃), we need to identify if each compound can react with sodium bicarbonate to release carbon dioxide (CO₂). The presence of CO₂ is what causes effervescence.
1. Identify the Compounds: The compounds given are:
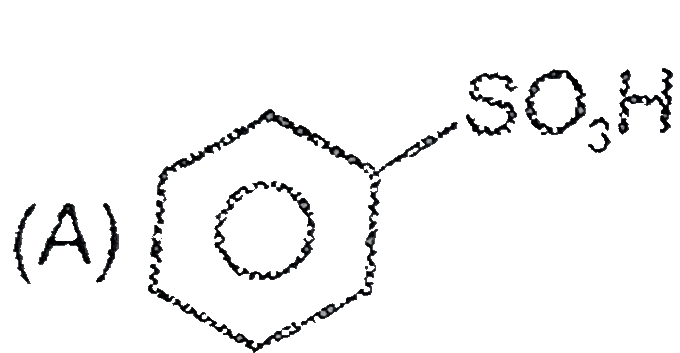
- Benzene sulfonic acid
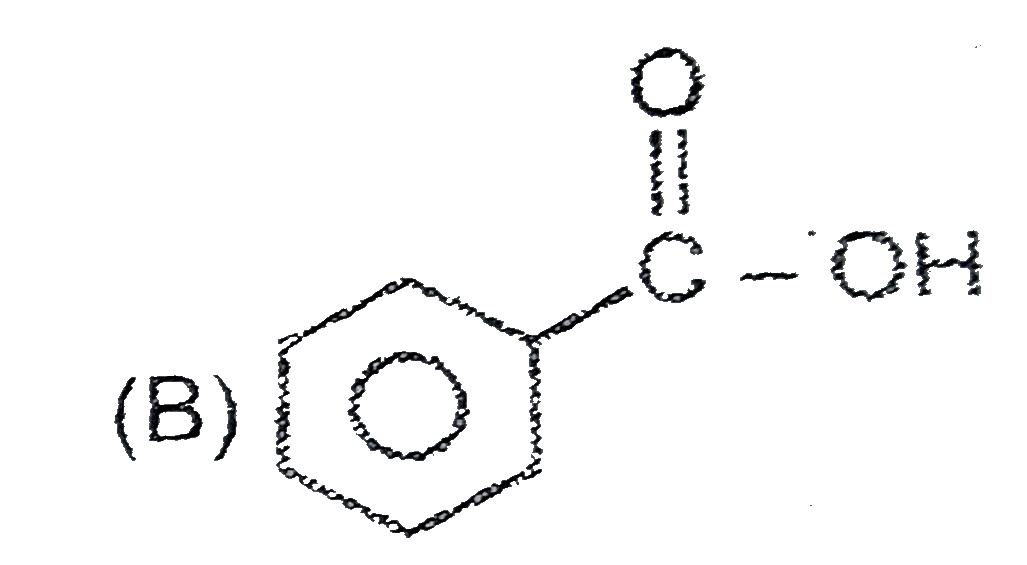
- Benzoic acid
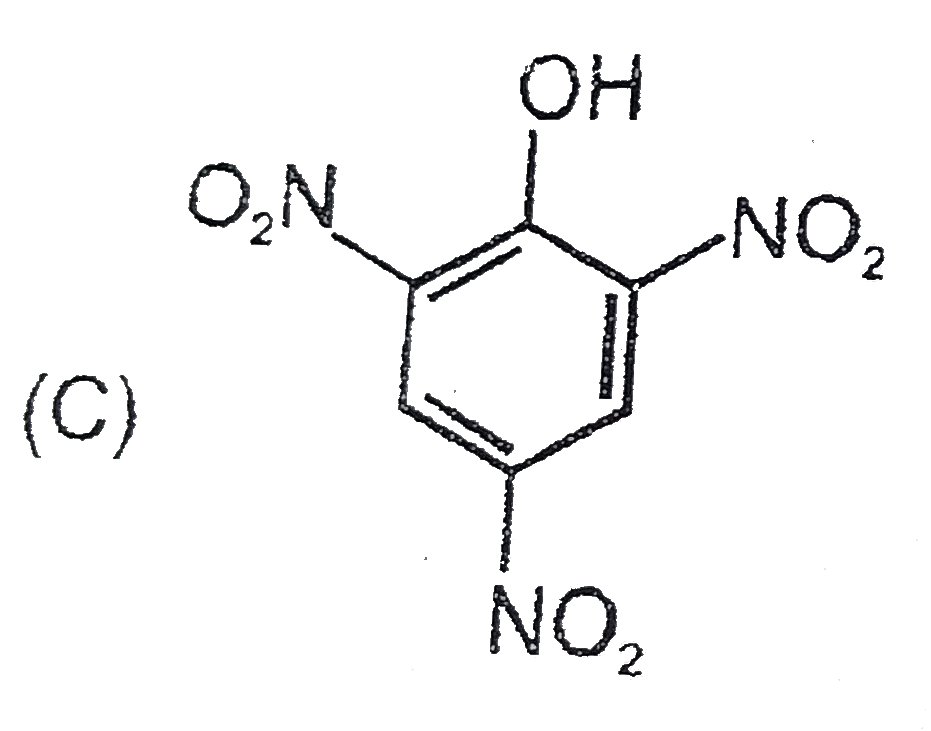
- Picric acid
- All of these
2. Understand the Reaction with Sodium Bicarbonate: Sodium bicarbonate is a weak base and reacts with acids to produce carbon dioxide gas, water, and a salt. The general reaction can be represented as:
Acid+NaHCO3→Salt+H2O+CO2(g)
3. Check Benzene Sulfonic Acid:
- Benzene sulfonic acid (C₆H₅SO₃H) can react with sodium bicarbonate:
C6H5SO3H+NaHCO3→C6H5SO3Na+H2O+CO2(g)
- Conclusion: This reaction produces CO₂, thus it will produce effervescence.
4. Check Benzoic Acid:
- Benzoic acid (C₆H₅COOH) also reacts with sodium bicarbonate:
C6H5COOH+NaHCO3→C6H5COONa+H2O+CO2(g)
- Conclusion: This reaction also produces CO₂, leading to effervescence.
5. Check Picric Acid:
- Picric acid (2,4,6-trinitrophenol) can react with sodium bicarbonate:
C6H3(NO2)3OH+NaHCO3→Salt+H2O+CO2(g)
- Conclusion: This reaction also produces CO₂, resulting in effervescence.
6. Final Conclusion:
- Since all three acids (benzene sulfonic acid, benzoic acid, and picric acid) react with sodium bicarbonate to produce CO₂, they all will produce effervescence.
- Therefore, the correct answer is D) All of these.
- Benzene sulfonic acid
- Benzoic acid
- Picric acid
- All of these
Acid+NaHCO3→Salt+H2O+CO2(g)
- Benzene sulfonic acid (C₆H₅SO₃H) can react with sodium bicarbonate:
C6H5SO3H+NaHCO3→C6H5SO3Na+H2O+CO2(g)
- Conclusion: This reaction produces CO₂, thus it will produce effervescence.
- Benzoic acid (C₆H₅COOH) also reacts with sodium bicarbonate:
C6H5COOH+NaHCO3→C6H5COONa+H2O+CO2(g)
- Conclusion: This reaction also produces CO₂, leading to effervescence.
- Picric acid (2,4,6-trinitrophenol) can react with sodium bicarbonate:
C6H3(NO2)3OH+NaHCO3→Salt+H2O+CO2(g)
- Conclusion: This reaction also produces CO₂, resulting in effervescence.
- Since all three acids (benzene sulfonic acid, benzoic acid, and picric acid) react with sodium bicarbonate to produce CO₂, they all will produce effervescence.
- Therefore, the correct answer is D) All of these.
Topper's Solved these Questions
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-I)
10 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise - 2 (Part-II)
8 Videos
- STRUCTURAL IDENTIFICATIONBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATIONEXERCISE - Exercise-1 (Part-I)
23 Videos
- STEREOISOMERISMBOOK - RESONANCE ENGLISHCHAPTER - STEREOISOMERISMEXERCISE - EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))
23 Videos
- SURFACE CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - SURFACE CHEMISTRYEXERCISE - Section - 5
1 Video
Similar Questions
Knowledge Check
One among the following compounds will not give effervescence with sodium carbonate:
AC6H5CO2HB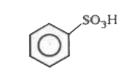 CC6H5OHD
CC6H5OHD
Which of the following reactions would produce same product?
A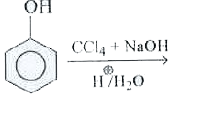 B
B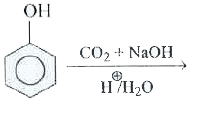 C
C D
D
Which among the following compounds will not give effervescence with sodium bicarbonate ?
View SolutionWhich of the following does not give effervescence with NaHCO3 ?
View SolutionDefine the following terms
Effervescence
View SolutionWhich of the following phenols is most soluble in aqueous sodium bicarbonate?
View SolutionWhich of the following would produce analog signals and which would produce digital signals?
View SolutionWhich of the following would produce analog signals and which would produce digital signals?
View Solution

