Consider the following six electronic configurations (remaining inner orbitals are completely filled) and mark the incorrect option.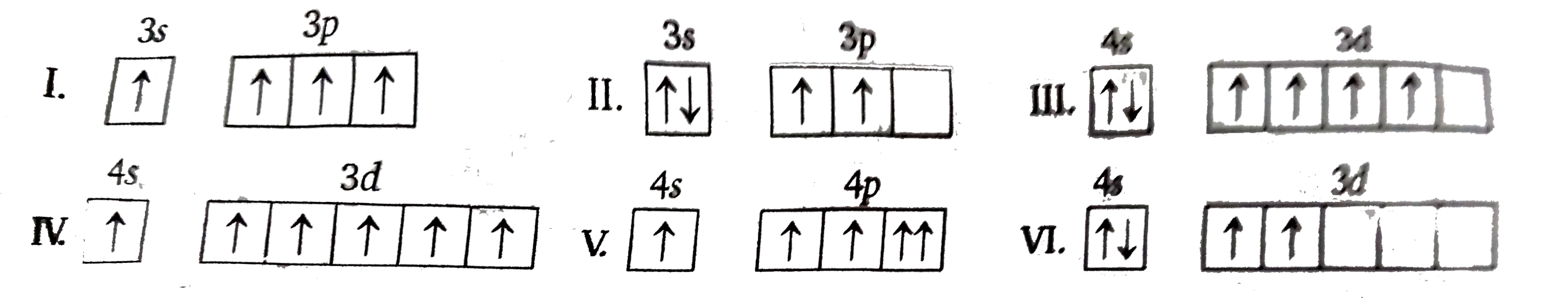
Stability order : II>I>IV>III
Order of spin multiplicity: IV>III=I>II
V does not violate all the three rules of electronic configuration
If VI represents A and A+ when kept near a magnet, acts as diamagnetic substance
The correct Answer is:A, B, C
A : excitation possible only in d-orbitals
B : Spin multiplicity =2|S|+1,|S|
=total spin
C : V violated Hund's rule
D : A+ is paramagnrtic due unpaired e−
∴ A, B, C are correct.
Topper's Solved these Questions
- NUCLEAR CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - NUCLEAR CHEMISTRYEXERCISE - PART-III
5 Videos
- NUCLEAR CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - NUCLEAR CHEMISTRYEXERCISE - STAGE-II
1 Video
- NUCLEAR CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - NUCLEAR CHEMISTRYEXERCISE - ADVANCED LEVEL PROBLEMS
30 Videos
- NITROGEN CONTAINING COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - NITROGEN CONTAINING COMPOUNDSEXERCISE - ORGANIC CHEMISTRY(Nitrogen containing Compounds)
30 Videos
- P BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - P BLOCK ELEMENTSEXERCISE - PART -II
23 Videos
Similar Questions
The set of quantum numbers, n = 2, l = 2, ml = 0 :
View SolutionHydrogen atom:
The electronic ground state of hydrogen atom contains one electron in the first orbit. If sufficient energy is provided, this electron can be promoted to higher energy levels. The electronic energy of a hydrogen-like species (any atom//ions with nuclear charge Z and one electron) can be given as
En=−RHZ2n2 where RH=Rydberg constant,n=principal quantum number
The energy in Joule of an electron in the second orbit of H- atom is:
View SolutionA 5g orbital has
View SolutionThe positive carbylamine test is not given by
View SolutionWrite the two important minerals of fluorine.
View SolutionThe inner shells of electrons are completely filled in
View SolutionIn the following six electronic configuration (remaining inner orbital are completely flled). Mark the correct optio(s).
View SolutionConsider the following six electronic configurations (remaining inner orbitals are completely filled) and mark the incorrect option.
View SolutionElectron configurations of few elements are given below,Mark the incorrect match.
View SolutionGive the outer orbit general electronic configuration of
(d) Inner transition elements.
View SolutionElectronic configuration of few elements are given below. Mark the incorrect match.
View Solutionकिसी तत्व के इलेक्ट्रॉनिक विन्यास में np6 के बाद निम्न कक्षक भरा जाता है
View SolutionWhich of the following electronic configuration is incorrect ?
View SolutionConsider the following six electronic configurations (remaining inner orbitals are completely filled) and mark the incorrect option.
View SolutionConsider the following six electronic configurations (remaining inner orbitals are completely filled) and mark the incorrect option

View SolutionElectronic configurations of few elements are given below, Mark the incorrect match.
View Solution

