Choose the correct statement (s) :
The shape of an atomic orbital depends upon azimuthal quantum number
The orientation of an atomic orbital depends upon the magnetic quantum number
The energy of an electron in an atomic orbital of multi-electron depends upon principal quantum number only
The number of degenerate atomic orbitals of one type depends upon the value of azimuthal quantum number
The correct Answer is:A, B, D
To solve the question, we need to analyze each statement regarding atomic orbitals and quantum numbers.
1. Understanding the Quantum Numbers:
- Principal Quantum Number (n): Indicates the energy level and size of the orbital. It can take positive integer values (1, 2, 3,...).
- Azimuthal Quantum Number (l): Determines the shape of the orbital. Its values range from 0 to (n-1). For example, if n=2, l can be 0 (s orbital) or 1 (p orbital).
- Magnetic Quantum Number (m): Specifies the orientation of the orbital in space. Its values range from -l to +l.
2. Analyzing Each Statement:
- Statement 1: "The shape of an atomic orbital depends upon azimuthal quantum number."
- This statement is correct because the azimuthal quantum number (l) determines the shape of the orbital (s, p, d, f).
- Statement 2: "The orientation of an atomic orbital depends upon magnetic quantum number."
- This statement is correct because the magnetic quantum number (m) defines the orientation of the orbital within a given shape.
- Statement 3: "The energy of an electron in an atomic orbital of a multi-electron atom depends upon principal quantum number only."
- This statement is incorrect. In multi-electron atoms, the energy of an electron also depends on the azimuthal quantum number (l). For example, in the second energy level (n=2), the 2s orbital has lower energy than the 2p orbital.
- Statement 4: "The number of degenerate atomic orbitals of one type depends upon the value of azimuthal quantum number only."
- This statement is correct. The number of degenerate orbitals (orbitals with the same energy) for a given l value is determined by the magnetic quantum number (m). For example, for l=1 (p orbitals), there are three degenerate orbitals corresponding to m = -1, 0, +1.
3. Conclusion:
- The correct statements are 1, 2, and 4.
Final Answer:
The correct statements are: 1, 2, and 4.
---
- Principal Quantum Number (n): Indicates the energy level and size of the orbital. It can take positive integer values (1, 2, 3,...).
- Azimuthal Quantum Number (l): Determines the shape of the orbital. Its values range from 0 to (n-1). For example, if n=2, l can be 0 (s orbital) or 1 (p orbital).
- Magnetic Quantum Number (m): Specifies the orientation of the orbital in space. Its values range from -l to +l.
- Statement 1: "The shape of an atomic orbital depends upon azimuthal quantum number."
- This statement is correct because the azimuthal quantum number (l) determines the shape of the orbital (s, p, d, f).
- Statement 2: "The orientation of an atomic orbital depends upon magnetic quantum number."
- This statement is correct because the magnetic quantum number (m) defines the orientation of the orbital within a given shape.
- This statement is incorrect. In multi-electron atoms, the energy of an electron also depends on the azimuthal quantum number (l). For example, in the second energy level (n=2), the 2s orbital has lower energy than the 2p orbital.
- This statement is correct. The number of degenerate orbitals (orbitals with the same energy) for a given l value is determined by the magnetic quantum number (m). For example, for l=1 (p orbitals), there are three degenerate orbitals corresponding to m = -1, 0, +1.
- The correct statements are 1, 2, and 4.
The correct statements are: 1, 2, and 4.
Topper's Solved these Questions
- NUCLEAR CHEMISTRYBook:RESONANCE ENGLISHChapter:NUCLEAR CHEMISTRYExercise:PART-III
Explore 5 Videos
- NUCLEAR CHEMISTRYBook:RESONANCE ENGLISHChapter:NUCLEAR CHEMISTRYExercise:STAGE-II
Explore 1 Video
- NUCLEAR CHEMISTRYBook:RESONANCE ENGLISHChapter:NUCLEAR CHEMISTRYExercise:ADVANCED LEVEL PROBLEMS
Explore 30 Videos
- NITROGEN CONTAINING COMPOUNDSBook:RESONANCE ENGLISHChapter:NITROGEN CONTAINING COMPOUNDSExercise:ORGANIC CHEMISTRY(Nitrogen containing Compounds)
Explore 30 Videos
- P BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:P BLOCK ELEMENTSExercise:PART -II
Explore 23 Videos
Similar Questions
The temperature coefficient of the cell is (∂E∂T)P. Choose the correct statements (s).
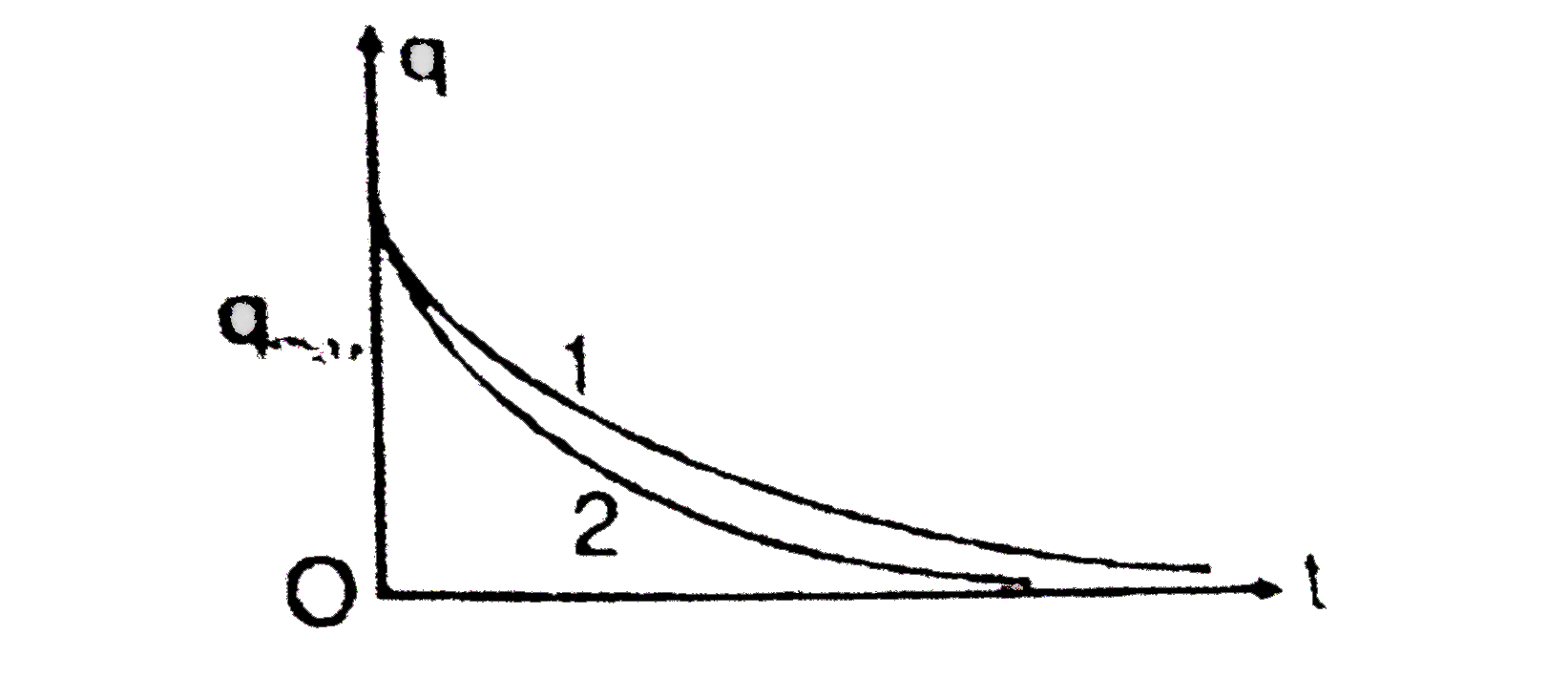
View SolutionThe instantaneous charge on capacitor in two discharging RC circuils is plotted with respect to time in figure. Choose the correct statement (s) (where E1 and E2 are emfs of two DC sources in two different charging circuits and capacitors are fully charged ).
View Solution
Knowledge Check
A group of people were surveyed one of the choose one of the tow TV shows they preferred more : Sherlock Holmes and Friends . Of the total 30 people who put forward their choice , 18 chose Sherlck Holmes and 20 chose Friends.Each preson had to choose one of the shows mentioned above . Choose the correct statements (s) :
I. 8 people liked both shows.
II. 22 people perferred one show over the other .
III. 12 people liked only one of the two shows.AOnly IBOnly IICOnly IIIDBoth I and II

In the adjacent figure blocks A and B of weight W each are lying at rest on a rough horizontal surface. They are pulled by applying forces F1 and F2 as shown in figure. When blocks are in limiting equilibrium F2=5F1 Choose the correct statement (s) (Assume same coefficient of friction for both the blocks)
View SolutionChoose the correct statements
View SolutionChoose the correct statements(s):
View SolutionConsider the following compounds:
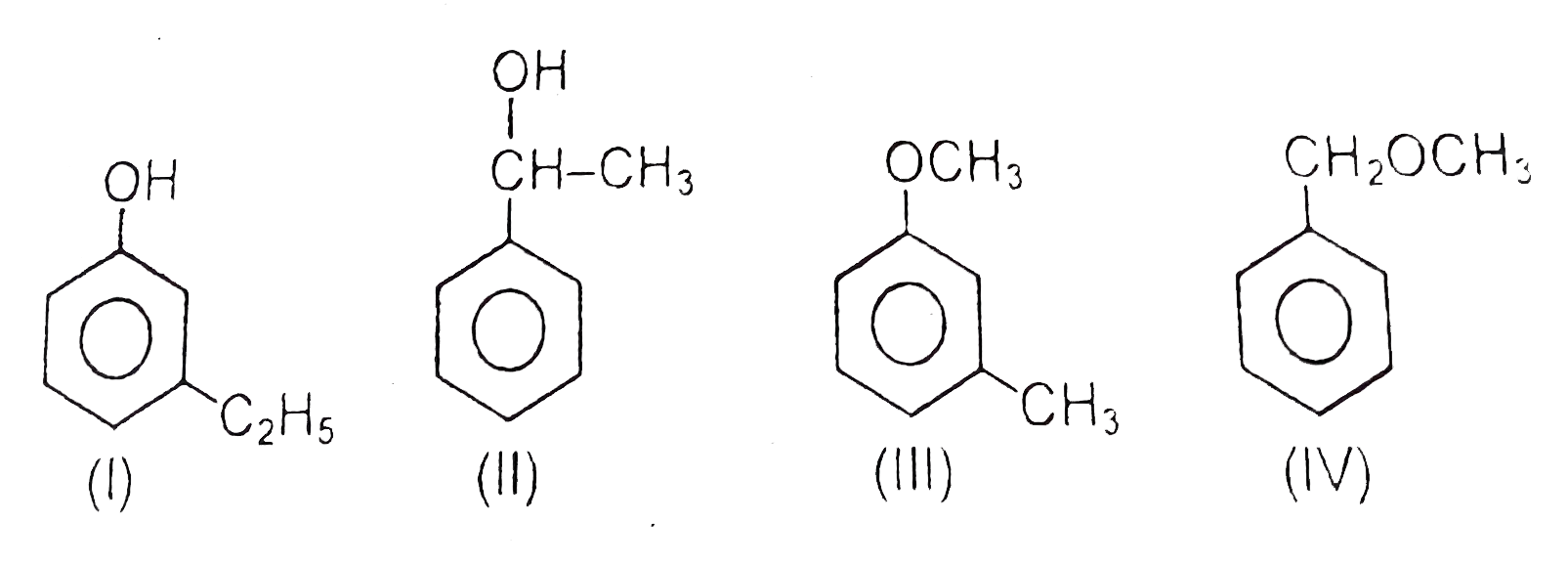
Choose the correct statements(s) from the following:
View Solution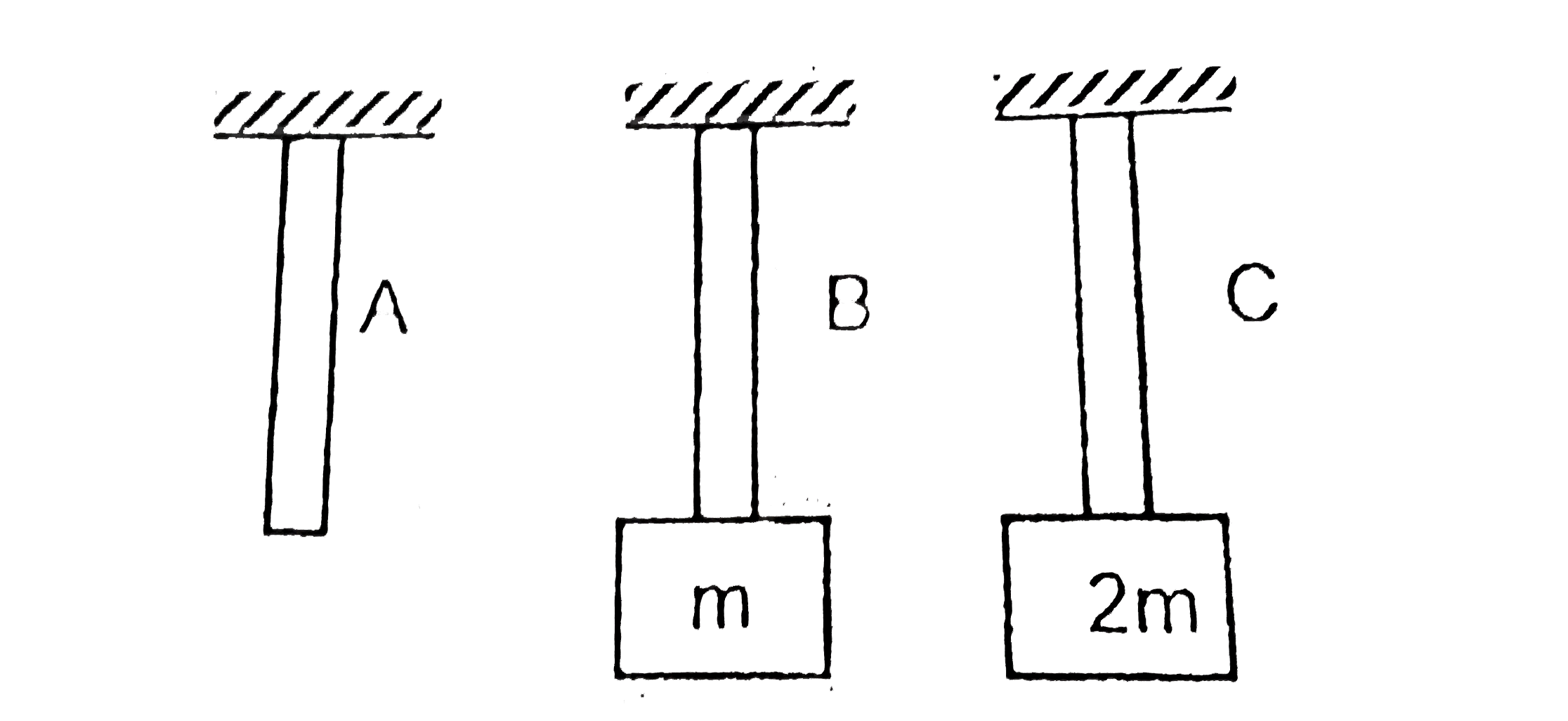
Consider the three ropes of same length and same linear density as (shown in figure) suspended from the ceiling.
A pulse is generated at the lowest point of the three ropes simultaneously. Choose the correct statements(s)
View SolutionA wave y= 3mm sin(2πx−200πt) is
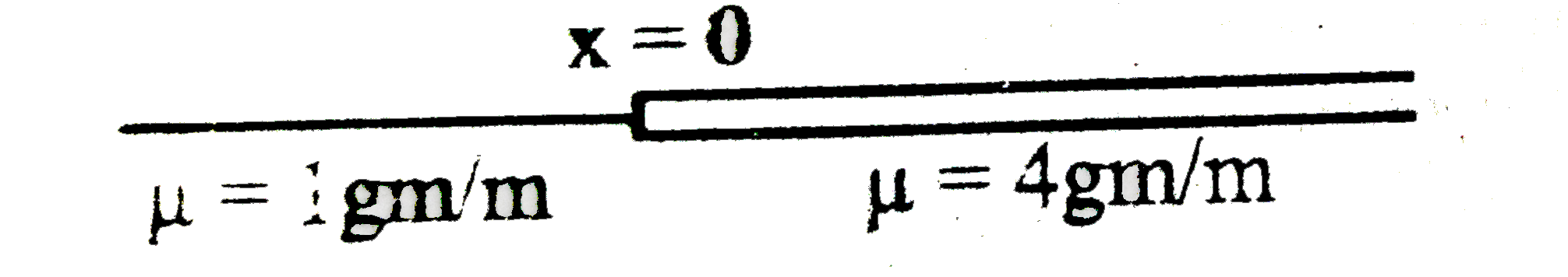
Propagating in the left string. It strikes the boundary at x=0 and is reflected partially and tranmitted partially. Choose the corrects statements (s)
View SolutionA block is thrown with a velocity of 2m/s−1 (relative to ground) on a belt, which is moving with velocity 4ms−1 in opposite direction of the initial velocity of block. If the block stops slipping on the belt after 4sec of the throwing then choose the correct statements (s)
View Solution
