Phenol is heated with a solution of mixture of KBr and KBrO3. The major product obtained in the above reaction is
2-Bromophenol
3-Bromophenol
4-Bromophenol
2,4,6-Tribromophenol
The correct Answer is:B
To solve the question regarding the reaction of phenol with a mixture of KBr and KBrO3, we can follow these steps:
1. Identify the Reactants:
- The reactants in this reaction are phenol (C6H5OH), potassium bromide (KBr), and potassium bromate (KBrO3).
2. Understand the Reaction Conditions:
- When phenol is heated with KBr and KBrO3, the bromate ion (BrO3^-) acts as an oxidizing agent, while the bromide ion (Br^-) provides bromine for substitution.
3. Mechanism of Reaction:
- The reaction involves electrophilic aromatic substitution where bromine (from KBr) substitutes the hydrogen atoms on the aromatic ring of phenol. Given that bromide ions are in excess, multiple bromination can occur.
4. Determine the Positions of Bromination:
- Phenol has an -OH group which is an activating group and directs electrophilic substitution to the ortho (2) and para (4) positions relative to itself. Therefore, bromination will occur predominantly at these positions.
5. Draw the Structure of the Product:
- The major product will have bromine atoms substituted at the ortho and para positions. Since there is an excess of bromide, we can expect bromination at all three positions: ortho (2), para (4), and meta (6).
6. Name the Product:
- The product formed is 2,4,6-tribromophenol, which has bromine substituents at the 2, 4, and 6 positions of the phenol ring.
7. Final Answer:
- The major product obtained from the reaction of phenol with KBr and KBrO3 is 2,4,6-tribromophenol.
- The reactants in this reaction are phenol (C6H5OH), potassium bromide (KBr), and potassium bromate (KBrO3).
- When phenol is heated with KBr and KBrO3, the bromate ion (BrO3^-) acts as an oxidizing agent, while the bromide ion (Br^-) provides bromine for substitution.
- The reaction involves electrophilic aromatic substitution where bromine (from KBr) substitutes the hydrogen atoms on the aromatic ring of phenol. Given that bromide ions are in excess, multiple bromination can occur.
- Phenol has an -OH group which is an activating group and directs electrophilic substitution to the ortho (2) and para (4) positions relative to itself. Therefore, bromination will occur predominantly at these positions.
- The major product will have bromine atoms substituted at the ortho and para positions. Since there is an excess of bromide, we can expect bromination at all three positions: ortho (2), para (4), and meta (6).
- The product formed is 2,4,6-tribromophenol, which has bromine substituents at the 2, 4, and 6 positions of the phenol ring.
- The major product obtained from the reaction of phenol with KBr and KBrO3 is 2,4,6-tribromophenol.
टॉपर्स ने हल किए ये सवाल
- AROMATIC COMPOUNDS Book:RESONANCE ENGLISHChapter:AROMATIC COMPOUNDS Exercise:JEE(MAIN) ONLINE PROBLEM
Explore 16 Videos
- AROMATIC COMPOUNDS Book:RESONANCE ENGLISHChapter:AROMATIC COMPOUNDS Exercise:PART-I PRACTICE TEST-1(IIT-JEE MAIN PATTERN)
Explore 30 Videos
- AROMATIC COMPOUNDS Book:RESONANCE ENGLISHChapter:AROMATIC COMPOUNDS Exercise:Exercise -3 Part-I
Explore 36 Videos
- ATOMIC STRUCTUREBook:RESONANCE ENGLISHChapter:ATOMIC STRUCTUREExercise:ORGANIC CHEMISTRY(Fundamental Concept )
Explore 16 Videos
समान प्रश्न
The following reaction :
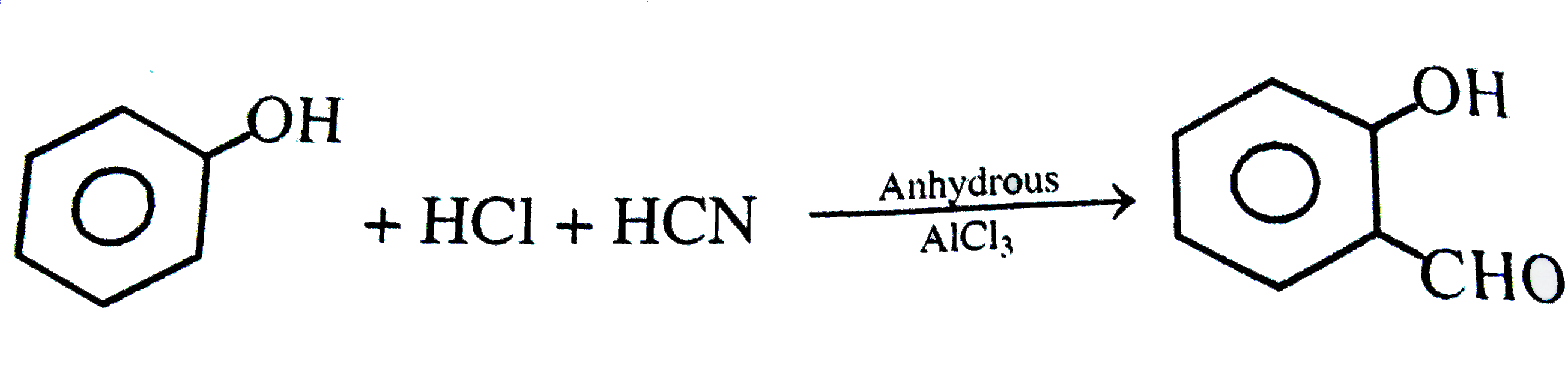
is known as
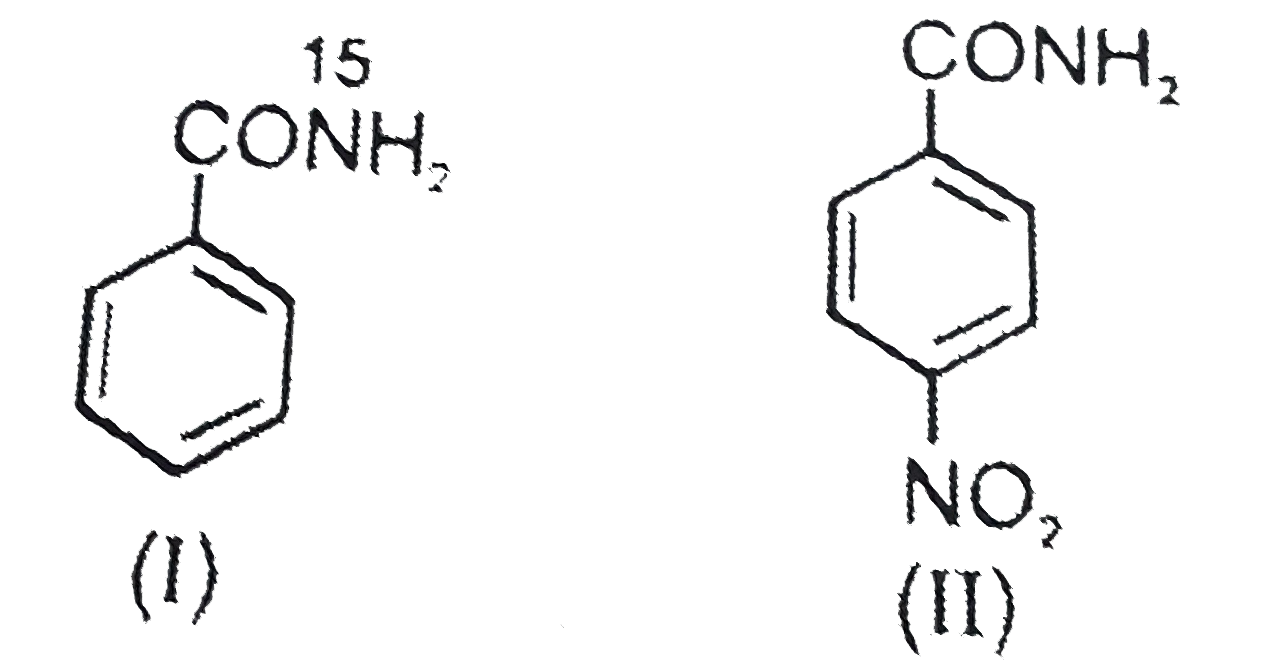
View SolutionThe products formed when a mixture of the following two amides( I and II) are treated with bromine and aqueous KOH are
View SolutionAssertion : In strongly acidic solution, aniline becomes less reactive towards electrophilic reagents .
Reason : Due to protonation of amino group the lone pair of electrons on nitrogen is not available for donation to benzene ring by resonance .
View SolutionThe magnetic moment of .25Mn in ionic state is √15B.M, then Mn is in:
View SolutionPhenol is heated wity a solution of mixture of KBr and KBrO_(3) . The major product obtained in the above reaction is
View SolutionPhenol is heated with a solution of mixture of KBr and KBrO_(3) . The major product obtained in the above reaction is
View SolutionPhenol is heated with a solution of mixture KBr and KBrO3. The major product obtained the above reaction is :
View SolutionPhenol is heated with a solution of mixture of KBr and KBrO_(3) . The major product obtained in the above reaction is
View Solutionफीनॉल को KBr तथा KBrO3 के मिश्रण के साथ गर्म किया गया। इस अभिक्रिया में उत्पन्न प्रमुख उत्पाद है:
View SolutionPhenol is heated with a solution of mixture of KBr and KBrO3. The major product obtained in the above reaction is
View SolutionPhenol is heated with a mixture of KBr and KBrO_(3) .
View SolutionPhenol is heated with an aqueous solution of Bromine. The major product obtained in the reaction is
View SolutionPhenol is heated with a mixture of KBr and KBrO_(3) . The major product obtained in the above reaction is:
View SolutionWhich major product is obtained on heating phenol with KBr and KBrO3 ?
View SolutionThe oxidation state of the product when 'KBr' and 'KBrO3' react in acidic medium is
View Solution
