In the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are
Four moles of NaOH and two moles of Br2
Two moles of NaOH and one mole of Br2
Four moles of NaOH and one mole of Br2
One mole of NaOH and mole of Br2
The correct Answer is:C
Step by step video solution for In the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br_2 used per mole of amine produced are by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - JEE(MAIN) ONLINE PROBLEM
16 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-I PRACTICE TEST-1(IIT-JEE MAIN PATTERN)
30 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - Exercise -3 Part-I
36 Videos
- ATOMIC STRUCTUREBOOK - RESONANCE ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - ORGANIC CHEMISTRY(Fundamental Concept )
16 Videos
Similar Questions
The following reaction :
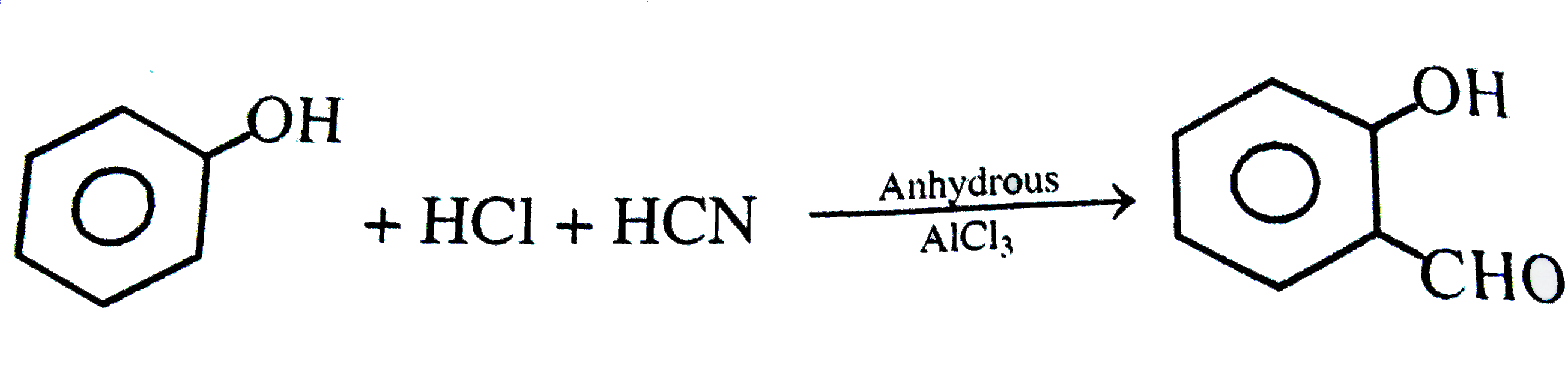
is known as
View SolutionThe products formed when a mixture of the following two amides( I and II) are treated with bromine and aqueous KOH are
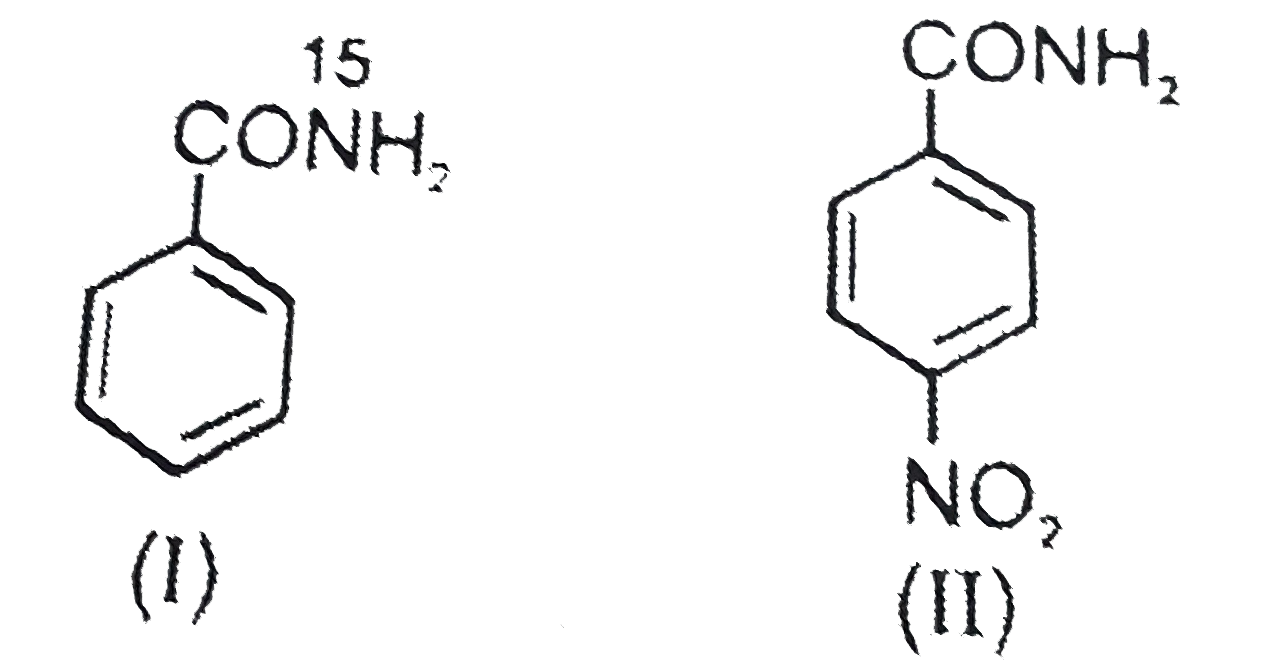
View SolutionAssertion : In strongly acidic solution, aniline becomes less reactive towards electrophilic reagents .
Reason : Due to protonation of amino group the lone pair of electrons on nitrogen is not available for donation to benzene ring by resonance .
View SolutionThe magnetic moment of .25Mn in ionic state is √15B.M, then Mn is in:
View SolutionRCONH2 is converted into RNH2 by means of Hofmann bromamide degradation.
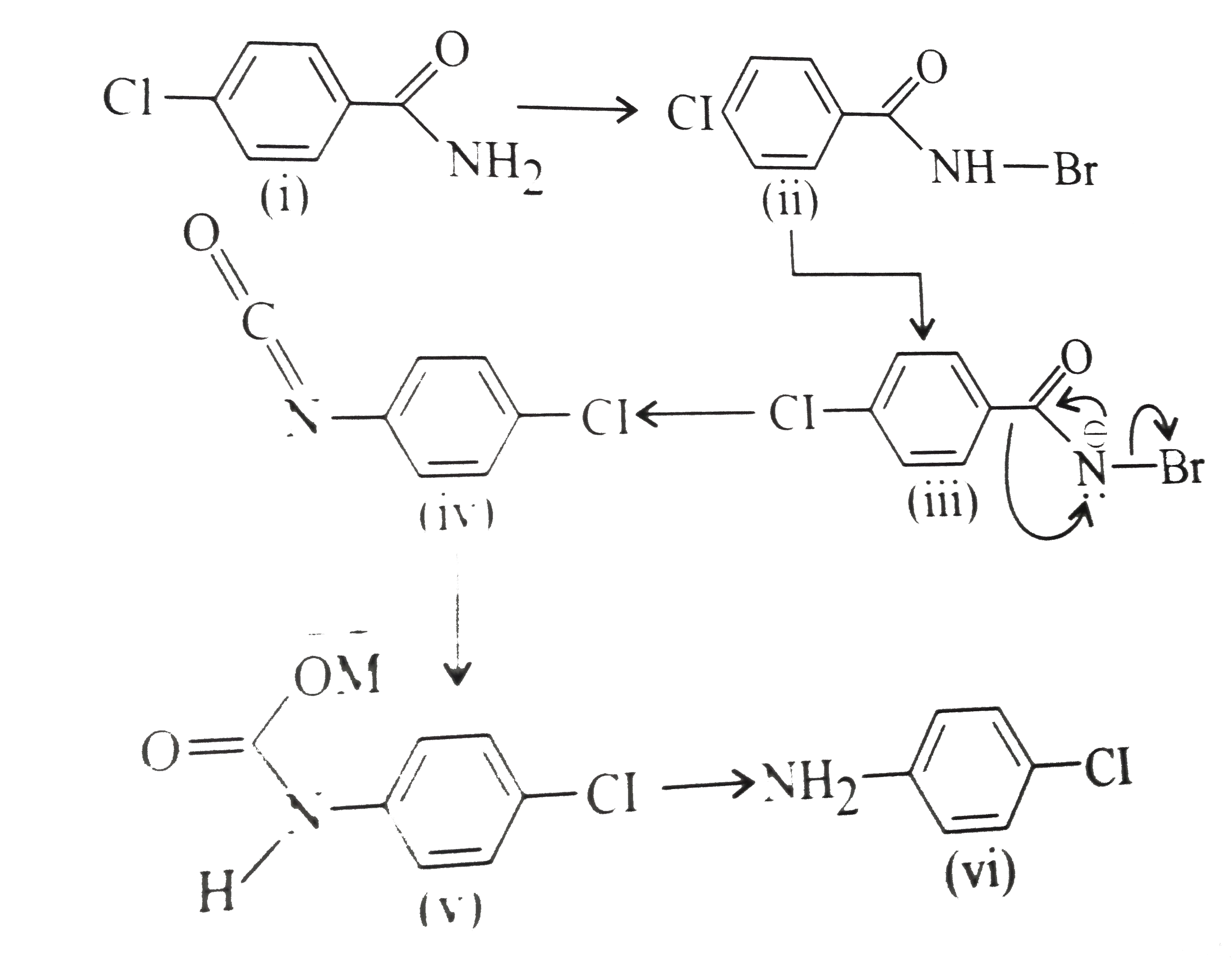
In this RCONHBr is formed form which this reaction has derived its name. Electron-donating group at phenyl activities the reaction. Hofmann degradation reaction is an intramolecular reaction.
Which is the rate-determining step in Hofmann bromamide degradation ?
View SolutionIn the hofmann-bromamide degradation reaction, the number of moles of NaOH and Br_(2) used per mole of amine produced are
View Solutionहॉफमान ब्रोमोऐमाइड निम्नीकरण विधि में NaOH तथा Br2 के प्रयुक्त के प्रयुक्त मोलों की संख्या प्रति मोल ऐमीन के बनने में होगी :-
View SolutionIn the hofmann-bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are
View SolutionWrite structure and IUPAC names of (i) The amide which gives propanamide by Hofmann bromamide reaction. (ii) the amine produce by the Hofmann degradation of benzamide.
View SolutionIn the hofmann-bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are
View SolutionIn the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br_2 used for mole of amine produced are-
View Solutionहॉफमान ब्रोमेमाइड निम्नीकरण अभिक्रिया में NaOH तथा Br2 के प्रयुक्त मोलों की संख्या प्रतिमोल ऐमीन के बनने में होगी
View SolutionWrite the reactions involved in the following: Hofmann bromamide degradation reaction
View SolutionThe most reactive compound towards Hofmann bromamide degradation is
View SolutionHow many moles of NaOH are consumed in the following Hofmann Bromamide reaction?
View SolutionAssertion : Hofmann degradation of benzamide gives aniline . Reason : Hofmann bromamide degradation reaction can be used for descending amine series .
View Solution

