Which of the following does not give effervescence with NaHCO3 ?
Phenol
p-Nitrophenol
2,4-dinitrophenol
2,4,6-trinitrophenol
The correct Answer is:A
To determine which of the following compounds does not give effervescence with sodium bicarbonate (NaHCO₃), we need to analyze the acidic properties of each compound listed. Sodium bicarbonate is a weak base and will react with strong acids to produce carbon dioxide (CO₂) gas, which is what causes effervescence.
1. Identify the Compounds:
The compounds given are:
- A) Phenol
- B) Para-nitrophenol
- C) 2,4-Dinitrophenol
- D) 2,4,6-Trinitrophenol
2. Understand the Nature of Sodium Bicarbonate:
Sodium bicarbonate (NaHCO₃) is a weak base. It will react with strong acids to release CO₂ gas, which results in effervescence.
3. Determine the Acidity of Each Compound:
- Phenol: A weak acid.
- Para-nitrophenol: Has a nitro group that is electron-withdrawing, making it a stronger acid than phenol.
- 2,4-Dinitrophenol: Contains two nitro groups, further increasing its acidity.
- 2,4,6-Trinitrophenol: Contains three nitro groups, making it a very strong acid.
4. Analyze the Reactivity with NaHCO₃:
- Since NaHCO₃ is a weak base, it will not react with weak acids.
- Among the compounds, phenol is the weakest acid, while the others (para-nitrophenol, 2,4-dinitrophenol, and 2,4,6-trinitrophenol) are stronger acids due to the presence of electron-withdrawing nitro groups.
5. Conclusion:
- Since phenol is a weak acid, it will not react with sodium bicarbonate to produce effervescence. Therefore, the answer to the question is A) Phenol.
Final Answer:
Phenol (Option A) does not give effervescence with NaHCO₃.
The compounds given are:
- A) Phenol
- B) Para-nitrophenol
- C) 2,4-Dinitrophenol
- D) 2,4,6-Trinitrophenol
Sodium bicarbonate (NaHCO₃) is a weak base. It will react with strong acids to release CO₂ gas, which results in effervescence.
- Phenol: A weak acid.
- Para-nitrophenol: Has a nitro group that is electron-withdrawing, making it a stronger acid than phenol.
- 2,4-Dinitrophenol: Contains two nitro groups, further increasing its acidity.
- 2,4,6-Trinitrophenol: Contains three nitro groups, making it a very strong acid.
- Since NaHCO₃ is a weak base, it will not react with weak acids.
- Among the compounds, phenol is the weakest acid, while the others (para-nitrophenol, 2,4-dinitrophenol, and 2,4,6-trinitrophenol) are stronger acids due to the presence of electron-withdrawing nitro groups.
- Since phenol is a weak acid, it will not react with sodium bicarbonate to produce effervescence. Therefore, the answer to the question is A) Phenol.
Phenol (Option A) does not give effervescence with NaHCO₃.
Topper's Solved these Questions
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I
31 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-III: PRACTICE TEST-2(IIT-JEE(ADVANCED pattern))
7 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - JEE(MAIN) ONLINE PROBLEM
16 Videos
- ATOMIC STRUCTUREBOOK - RESONANCE ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - ORGANIC CHEMISTRY(Fundamental Concept )
16 Videos
Similar Questions
Compound A is chiral and has the molecular formula C8H11N. When A reacts nitrous acid then occurs a brisk evolution of N2 gas. A dissolves in aqueous HCl. What is the identity of A ?
View Solution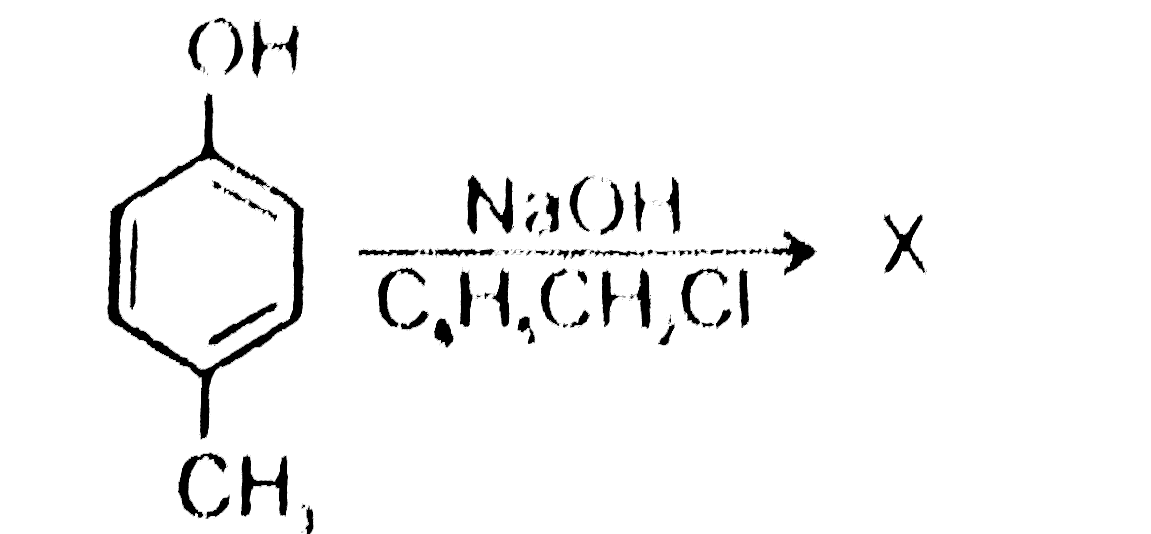
The major product X in the above reaction is
View SolutionThe following reaction :
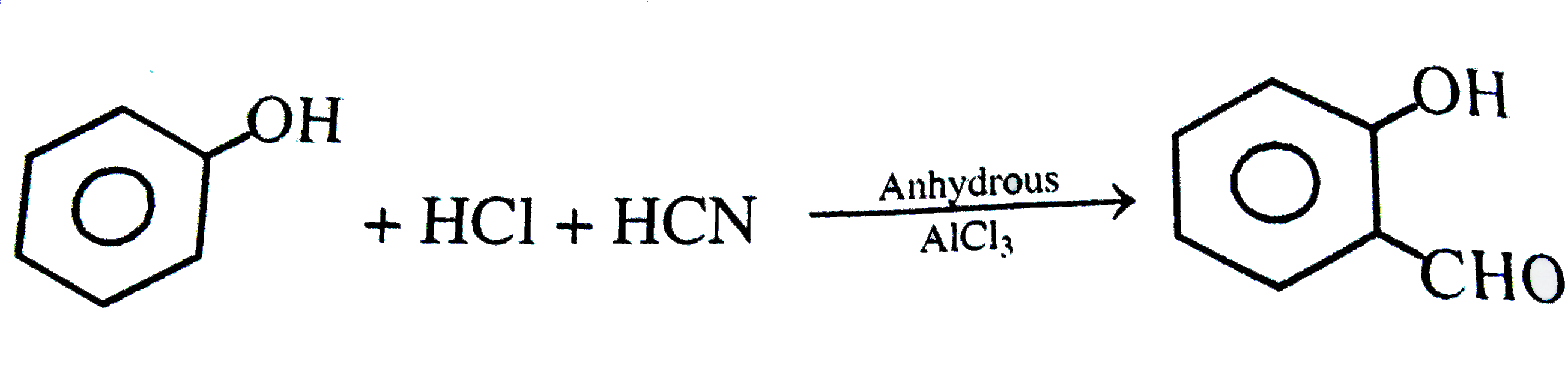
is known as
View SolutionThe magnetic moment of .25Mn in ionic state is √15B.M, then Mn is in:
View SolutionWhich of the following compounds gives carbondioxide with NaHCO3?
View SolutionWhich of the following will give effervesce of CO2 with NaHCO3
View SolutionWhich derivative of phenol gives effervescence with NaHCO_(3) ?
View SolutionAmong the following compounds, the one (s) that gives (gives) effervescence with aqueous NaHCO_(3) solution is (are) : (I) (CH_(3)CO)_(2)O , (II) CH_(3)COOH (III) PhOH , (IV) CH_(3)COCHO
View SolutionCompound that fails to give effervescence with NaHCO_(3) is
View SolutionWhich one of the following pairs gives effervescence with aq. NaHCO3?
I. CH3COCl II. CH3COCH3
III. CH3COOCH3 IV. CH3COOCOCH3
View SolutionName the functional group present in an organic compound which gives brisk effervescence with NaHCO_(3)
View SolutionWhich derivative of phenol gives effervescence with NaHCO_(3) -
View SolutionWhich of the following does not give effervescence with NaHCO3 ?
View SolutionWhich one of the following pairs gives effervescence with aq. NaHCO_(3) ?
View Solutionwhich of the following amine will not give effervescence with HNO_(2)
View Solution

