Aniline can be obtained by reduction of nitrobenzene with
Fe/HCl
Sn/NaOH
Electrolytic reduction under strong acidic conditions
All of these
The correct Answer is:A
To solve the question "Aniline can be obtained by reduction of nitrobenzene with," we will analyze the options provided and determine which one is correct.
1. Identify the Compound: We start with nitrobenzene (C6H5NO2), which contains a nitro group (-NO2) attached to a benzene ring.
2. Understand the Reduction Process: The reduction of nitrobenzene involves converting the nitro group (-NO2) to an amine group (-NH2), which is the structure of aniline (C6H5NH2).
3. Evaluate the First Option: Fe and HCl:
- Iron (Fe) in the presence of hydrochloric acid (HCl) acts as a strong reducing agent.
- This combination can effectively reduce nitrobenzene to aniline.
- The reaction can be summarized as:
C6H5NO2+3H2→C6H5NH2+2H2O
- Thus, this option is valid.
4. Evaluate the Second Option: Sn and NaOH:
- Tin (Sn) is a reducing agent, but when used in a basic medium (NaOH), it does not effectively reduce nitrobenzene.
- Therefore, this option is incorrect.
5. Evaluate the Third Option: Electrolytic Reduction under Strong Acidic Conditions:
- Although electrolytic reduction can reduce nitrobenzene, it typically requires weak acidic conditions, not strong acidic conditions.
- Therefore, this option is also incorrect.
6. Conclusion: The only correct method for reducing nitrobenzene to aniline from the given options is using Fe and HCl.
Final Answer:
Aniline can be obtained by reduction of nitrobenzene with Fe and HCl.
---
- Iron (Fe) in the presence of hydrochloric acid (HCl) acts as a strong reducing agent.
- This combination can effectively reduce nitrobenzene to aniline.
- The reaction can be summarized as:
C6H5NO2+3H2→C6H5NH2+2H2O
- Thus, this option is valid.
- Tin (Sn) is a reducing agent, but when used in a basic medium (NaOH), it does not effectively reduce nitrobenzene.
- Therefore, this option is incorrect.
- Although electrolytic reduction can reduce nitrobenzene, it typically requires weak acidic conditions, not strong acidic conditions.
- Therefore, this option is also incorrect.
Aniline can be obtained by reduction of nitrobenzene with Fe and HCl.
Topper's Solved these Questions
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I
31 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-III: PRACTICE TEST-2(IIT-JEE(ADVANCED pattern))
7 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - JEE(MAIN) ONLINE PROBLEM
16 Videos
- ATOMIC STRUCTUREBOOK - RESONANCE ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - ORGANIC CHEMISTRY(Fundamental Concept )
16 Videos
Similar Questions
Knowledge Check
Birch reduction of nitrobenzene will produce :
A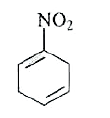 B
B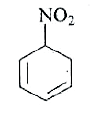 C
C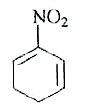 D
D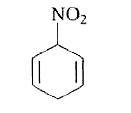
Which of the following give aniline by reduction of nitrobenzene?
AH2/Pd−CBSn+HClCFe+HClDLiAlH4
Aniline can be prepares by :
View SolutionProduct obtained by electrolytic reduction of nitrobenzene in presence of H2SO4 s
View SolutionThe product obtained by reduction of benzyl bromide with LIAIH4 is
View SolutionPrimary alcohols can be obtained can be obtained from the reaction of the RMgX with
View SolutionWhich of the following products can be obtained by reductive ozonolysis of o-xylene ?
View SolutionAlkali metals can be obtained by chemical reduction of their compounds.
View Solution

