In the following reaction sequence Y is,
PhOCOCH3AlCl3−−→Δ(X)I2−−−→NaOH(Y)
PhCOONa
PhCOOH

The correct Answer is:D
To solve the problem step by step, we will analyze the reaction sequence provided and deduce the structure of compound Y.
Step 1: Identify the starting compound
The starting compound is PhOCOCH3, which is a phenyl group (Ph) attached to a carbonyl (C=O) and a methyl group (CH3).
Step 2: Reaction with AlCl3 and heat
When PhOCOCH3 is treated with AlCl3 and heat, it undergoes a Friedel-Crafts acylation reaction. The lone pair of electrons on the oxygen atom of the carbonyl group coordinates with AlCl3, forming a complex. This activates the carbonyl carbon for electrophilic attack.
Intermediate structure:
- The structure can be represented as:
PhO−C(=O)−CH3
After coordination with AlCl3, the carbonyl carbon becomes more electrophilic.
Step 3: Formation of the acyloxybenzene
The acyloxybenzene is formed after the loss of CH3C≡O (acetyl group) as a leaving group. The resulting structure after the rearrangement will be:
Ph−O−C(=O)−CH3
This structure has a negative charge on the oxygen after the rearrangement.
Step 4: Reaction with I2 and NaOH
The next step involves treating the product with I2 and NaOH. This is indicative of the iodoform reaction, which occurs with methyl ketones or compounds that can generate a methyl ketone.
1. The negative charge on the oxygen can facilitate the formation of a hydroxyl group.
2. The iodoform reaction will convert the methyl group adjacent to the carbonyl into iodoform (CHI3) and leave behind a carboxylate ion.
Final product structure:
The final product Y will be:
Ph−O−C(=O)−Na
Where the sodium ion is associated with the carboxylate group.
Conclusion
Thus, the final product Y is PhOCOONa (sodium phenyl carbonate).
The starting compound is PhOCOCH3, which is a phenyl group (Ph) attached to a carbonyl (C=O) and a methyl group (CH3).
When PhOCOCH3 is treated with AlCl3 and heat, it undergoes a Friedel-Crafts acylation reaction. The lone pair of electrons on the oxygen atom of the carbonyl group coordinates with AlCl3, forming a complex. This activates the carbonyl carbon for electrophilic attack.
- The structure can be represented as:
PhO−C(=O)−CH3
After coordination with AlCl3, the carbonyl carbon becomes more electrophilic.
The acyloxybenzene is formed after the loss of CH3C≡O (acetyl group) as a leaving group. The resulting structure after the rearrangement will be:
Ph−O−C(=O)−CH3
This structure has a negative charge on the oxygen after the rearrangement.
The next step involves treating the product with I2 and NaOH. This is indicative of the iodoform reaction, which occurs with methyl ketones or compounds that can generate a methyl ketone.
2. The iodoform reaction will convert the methyl group adjacent to the carbonyl into iodoform (CHI3) and leave behind a carboxylate ion.
The final product Y will be:
Ph−O−C(=O)−Na
Where the sodium ion is associated with the carboxylate group.
Thus, the final product Y is PhOCOONa (sodium phenyl carbonate).
টপার এই প্রশ্নগুলো সমাধান করেছে
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-II: NATIONAL STANDARD EXAMINATION IN CHEMISTRY (NSEC) STAGE-I
31 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - PART-III: PRACTICE TEST-2(IIT-JEE(ADVANCED pattern))
7 Videos
- AROMATIC COMPOUNDS BOOK - RESONANCE ENGLISHCHAPTER - AROMATIC COMPOUNDS EXERCISE - JEE(MAIN) ONLINE PROBLEM
16 Videos
- ATOMIC STRUCTUREBOOK - RESONANCE ENGLISHCHAPTER - ATOMIC STRUCTUREEXERCISE - ORGANIC CHEMISTRY(Fundamental Concept )
16 Videos
সম্পর্কিত প্রশ্ন
Compound A is chiral and has the molecular formula C8H11N. When A reacts nitrous acid then occurs a brisk evolution of N2 gas. A dissolves in aqueous HCl. What is the identity of A ?
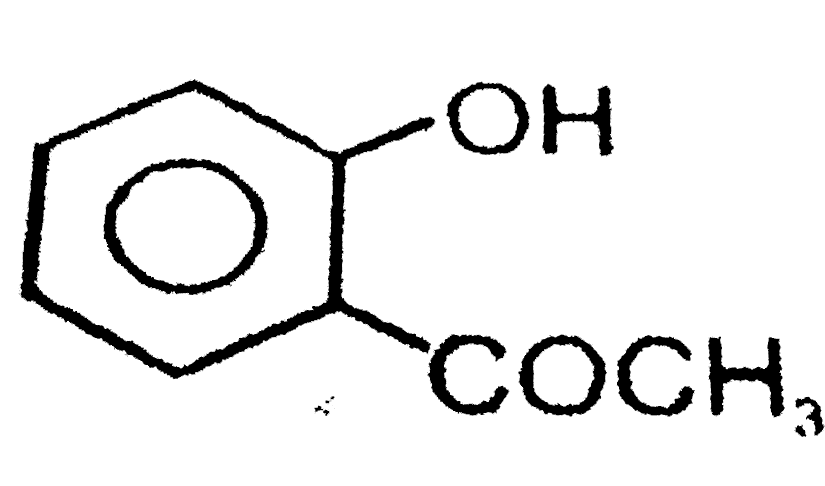
View Solution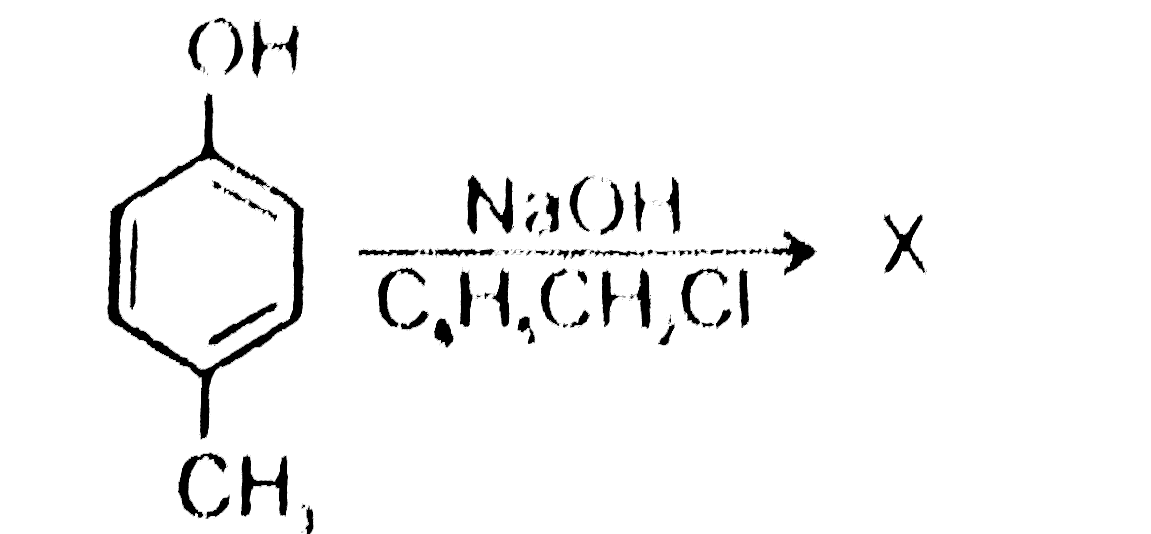
The major product X in the above reaction is
View SolutionThe following reaction :
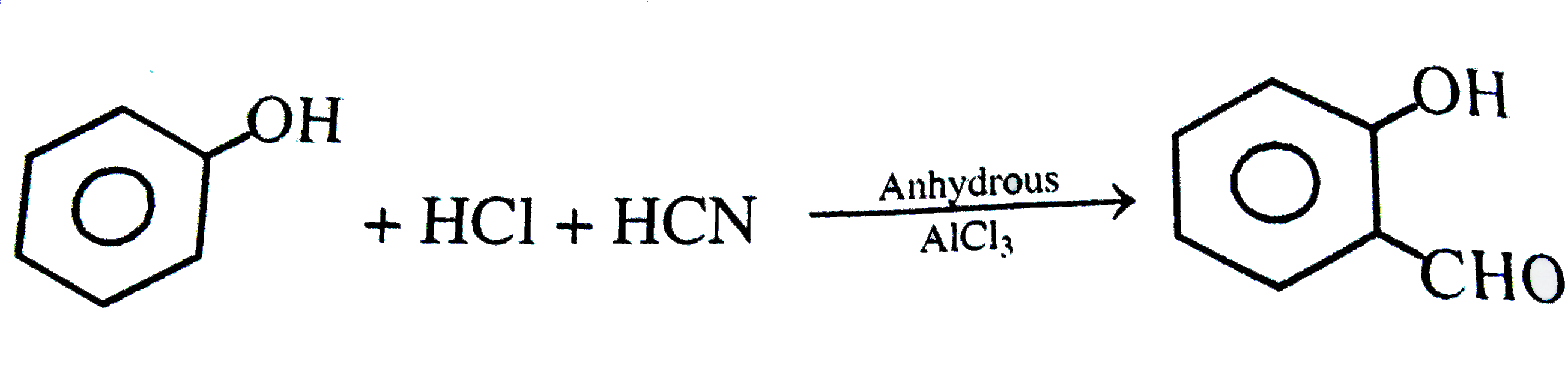
is known as
View SolutionThe magnetic moment of .25Mn in ionic state is √15B.M, then Mn is in:
View SolutionCH_(3)-CH_(2)-underset(CH_(3))underset(|)overset(CH_(3))overset(|)C-underset(OH)underset(|)(CH)-CD_(3)underset(Delta)overset(H_(3)PO_(4))rarr Total no. of alkenes (X) underset(distilation)overset(Fractional)rarr No. of fractions obtained (Y) . X and Y are:
View Solution , AlCl3−−→Δ Product formed as?
, AlCl3−−→Δ Product formed as?
View SolutionIn the given reaction sequence C6H5−CH2−NH2CHCl3/Alc.KOH−−−−−−−−−−−→Δ[X]H2O/NaOH−−−−−−−→[Y],[Y] will be:
View SolutionIn the given reaction :
Ph−C∣∣O−CHY3H2N−OH−−−−−−→(X)PCI5−−→Δ(Y)
Y will be
View Solution(CH_(3))_(2) CO underset((HCl))overset(NaCN)rarr X underset(Delta)overset(H_(3)O^(+))rarr Y In the above sequence of reactions X and Y are
View SolutionEthanol overset(P_(4)//I_(2))rarr X overset((i)KOH(alc))underset((ii) HBr)rarr Y In this sequence of reactions Y is :
View SolutionIn the reaction
CH3CN+2HHCl−−→etherXH2O−−→ΔY, Y is
View SolutionIn the following reaction sequence Y is,
PhOCOCH3AlCl3−−→Δ(X)I2−−−→NaOH(Y)
View SolutionConsider the following sequence of reactions
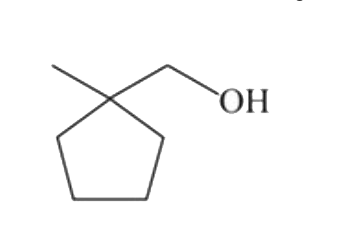 H+/Δ−−−−→(X)1.03−−−−−−−−−−→2.Zn/CH3COOH(Y)NaOH−−−→Δ
H+/Δ−−−−→(X)1.03−−−−−−−−−−→2.Zn/CH3COOH(Y)NaOH−−−→Δ 
The intermediate products (X) and (Y) are respectively
View SolutionThe 'Y' in the following sequence of reactions CH3−CH∣OH−CH3PBr3−−→(X)aq.NaOH−−−−−→(Y) is
View SolutionHexane Cr2O3−−−−→773K10−20atmXCH3COCl−−−−−−−−−→AnyhdrousAlCl3Y Y in the above sequence of reaction is
View Solution

