Which is most basic among the followings ?
Ph−NH2
NH3
CH3−NH2
C2H5−CN
The correct Answer is:C
To determine which compound is the most basic among the given options, we will analyze the basicity of each compound step by step.
Step 1: Identify the Compounds
The compounds given are:
1. pH NH2 (aniline)
2. NH3 (ammonia)
3. CH3 NH2 (methylamine)
4. C2H5 CN (ethanenitrile)
Step 2: Classify the Compounds
- pH NH2 (aniline) is a primary amine.
- NH3 (ammonia) is a simple amine.
- CH3 NH2 (methylamine) is also a primary amine.
- C2H5 CN (ethanenitrile) is not an amine; it contains a cyanide group.
Step 3: Eliminate Non-Amine Compound
Since C2H5 CN is not an amine, we can eliminate it from consideration. This leaves us with:
1. pH NH2 (aniline)
2. NH3 (ammonia)
3. CH3 NH2 (methylamine)
Step 4: Compare Basicity of Ammonia and Primary Amines
It is known that primary amines (like CH3 NH2 and pH NH2) are generally more basic than ammonia (NH3). Thus, we can eliminate ammonia from being the most basic.
Step 5: Compare Basicity of pH NH2 and CH3 NH2
Now we need to compare the basicity of the two primary amines:
- pH NH2 (aniline) has a phenyl group (pH) attached to the nitrogen. The phenyl group is electron-withdrawing due to resonance, which delocalizes the lone pair of electrons on nitrogen, making them less available for protonation.
- CH3 NH2 (methylamine) has a methyl group (CH3) attached to the nitrogen. The methyl group is electron-donating, which increases the availability of the lone pair of electrons on nitrogen for protonation.
Step 6: Conclusion
Since the methyl group increases the basicity while the phenyl group decreases it, CH3 NH2 (methylamine) is more basic than pH NH2 (aniline). Therefore, the most basic compound among the given options is:
Answer: CH3 NH2 (methylamine)
---
The compounds given are:
1. pH NH2 (aniline)
2. NH3 (ammonia)
3. CH3 NH2 (methylamine)
4. C2H5 CN (ethanenitrile)
- pH NH2 (aniline) is a primary amine.
- NH3 (ammonia) is a simple amine.
- CH3 NH2 (methylamine) is also a primary amine.
- C2H5 CN (ethanenitrile) is not an amine; it contains a cyanide group.
Since C2H5 CN is not an amine, we can eliminate it from consideration. This leaves us with:
1. pH NH2 (aniline)
2. NH3 (ammonia)
3. CH3 NH2 (methylamine)
It is known that primary amines (like CH3 NH2 and pH NH2) are generally more basic than ammonia (NH3). Thus, we can eliminate ammonia from being the most basic.
Now we need to compare the basicity of the two primary amines:
- pH NH2 (aniline) has a phenyl group (pH) attached to the nitrogen. The phenyl group is electron-withdrawing due to resonance, which delocalizes the lone pair of electrons on nitrogen, making them less available for protonation.
- CH3 NH2 (methylamine) has a methyl group (CH3) attached to the nitrogen. The methyl group is electron-donating, which increases the availability of the lone pair of electrons on nitrogen for protonation.
Since the methyl group increases the basicity while the phenyl group decreases it, CH3 NH2 (methylamine) is more basic than pH NH2 (aniline). Therefore, the most basic compound among the given options is:
টপার এই প্রশ্নগুলো সমাধান করেছে
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Part-II: NSEC (Stage-I)
Explore 45 Videos
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Part-III: Practice Test-2 (IIT-JEE (Advanced Pattern))
Explore 13 Videos
- GENERAL ORGANIC CHEMISTRY IIBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY IIExercise:Exercise-3 Part:II JEE(MAIN) ONLINE PROBLEMS
Explore 16 Videos
- GASEOUS STATEBook:RESONANCE ENGLISHChapter:GASEOUS STATEExercise:ORGANIC CHEMISTRY(Hydrocarbon)
Explore 17 Videos
- GENERAL ORGANIC CHEMISTRY-IBook:RESONANCE ENGLISHChapter:GENERAL ORGANIC CHEMISTRY-IExercise:PART-III : PRACTICE TEST-19
Explore 1 Video
সম্পর্কিত প্রশ্ন
Which is the following is the strongest acid ?
View SolutionThe decreasing order of stability of following cations is :-
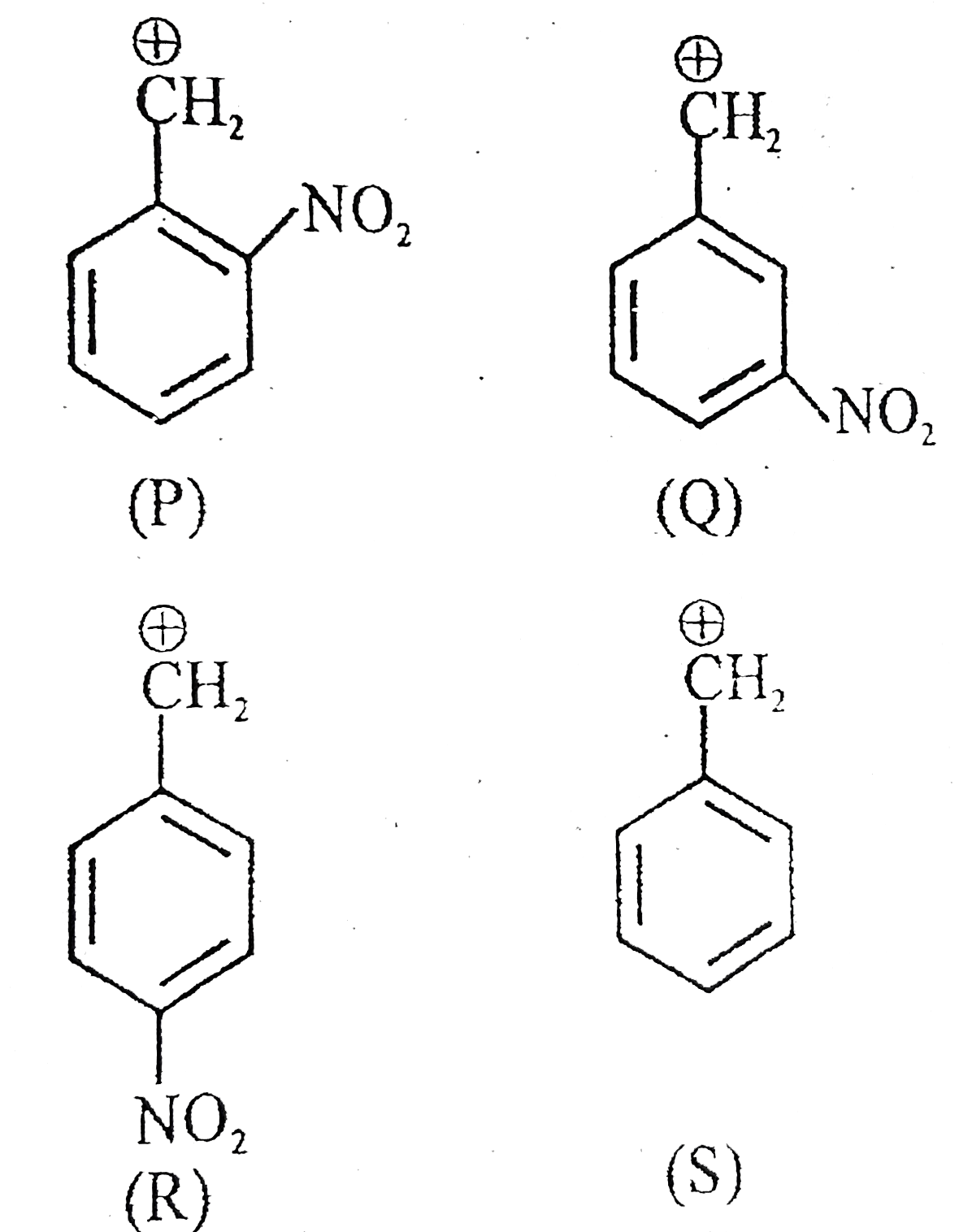
View SolutionIn which of the following pairs A is more stable than B?
View SolutionA certain quantity of a gas occupied 100ml when collected over water at 15∘C and 750mm pressure . It occupies 91.9ml in dry state at NTP. Find the V.P. of water at 15∘C
View SolutionMatch the column.
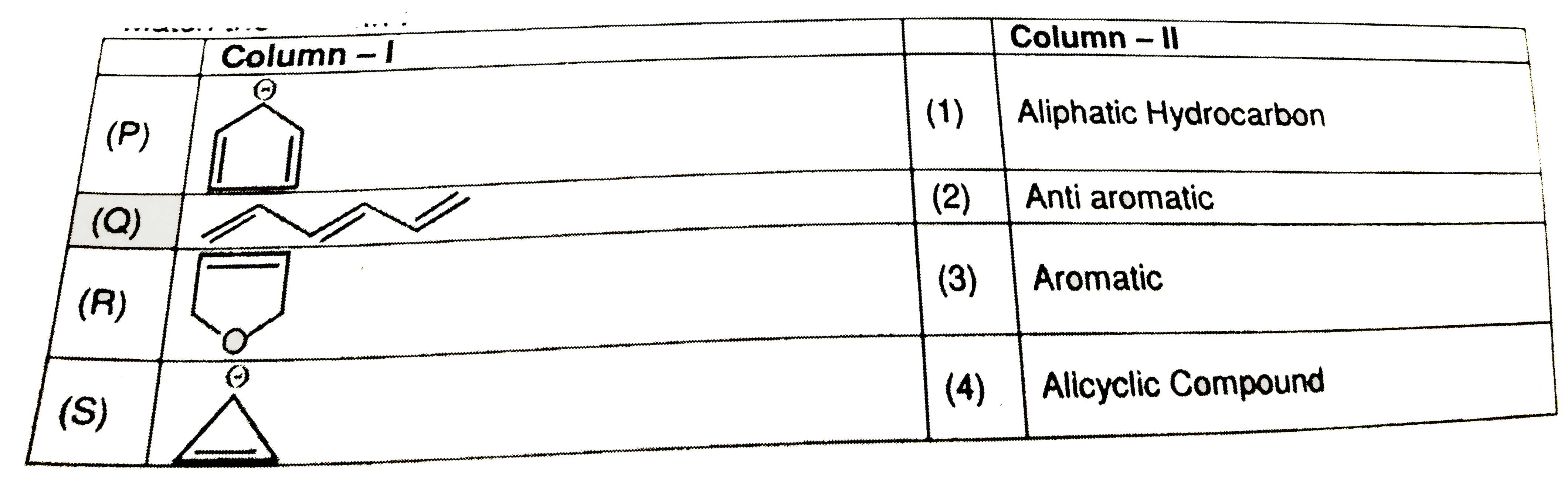
View SolutionWhich one among the following is the most basic?
View SolutionAmong the following the most basic is
View SolutionAmong the following which one is most basic-
View SolutionWhich is the most basic among the following?
View SolutionAmong the following oxides, the one which is most basic is
View SolutionAmong the following, the one which is the most basic is
View SolutionWhich is most basic among the followings ?
View SolutionAmong the following metal oxides, which is most basic?
View SolutionWhich is most basic among the following?
View SolutionMost basic among the following
View SolutionMost basic among the following
View Solution
