The reagent used for Friedel-Craft's reaction is :
Dry ether
AlCl3
Anhydrous AlCl3
P2O5
The correct Answer is:C
To answer the question regarding the reagent used for Friedel-Craft's reaction, we can break down the solution into clear steps:
1. Understanding Friedel-Craft's Reaction:
- Friedel-Craft's reaction is a type of electrophilic aromatic substitution where an alkyl group is introduced into an aromatic ring (like benzene).
2. Identifying the Reagents:
- The reaction typically involves benzene and an alkyl halide (like methyl chloride or ethyl chloride).
- A catalyst is required to facilitate the reaction, which is usually an aluminum chloride (AlCl3).
3. Role of Aluminum Chloride:
- In the presence of anhydrous aluminum chloride (AlCl3), the alkyl halide reacts to generate a carbocation (an electrophile).
- For example, when methyl chloride (CH3Cl) reacts with AlCl3, it forms a methyl cation (CH3+) and AlCl4-.
4. Electrophilic Attack:
- The generated carbocation (CH3+) then attacks the benzene ring, substituting one of the hydrogen atoms and forming an alkyl-substituted benzene (like toluene).
5. Final Product:
- The overall reaction results in the formation of an alkyl benzene and a hydrogen halide (HCl in the case of methyl chloride).
6. Conclusion:
- Therefore, the reagent used for Friedel-Craft's reaction is anhydrous aluminum chloride (AlCl3).
Final Answer:
The reagent used for Friedel-Craft's reaction is anhydrous AlCl3.
---
- Friedel-Craft's reaction is a type of electrophilic aromatic substitution where an alkyl group is introduced into an aromatic ring (like benzene).
- The reaction typically involves benzene and an alkyl halide (like methyl chloride or ethyl chloride).
- A catalyst is required to facilitate the reaction, which is usually an aluminum chloride (AlCl3).
- In the presence of anhydrous aluminum chloride (AlCl3), the alkyl halide reacts to generate a carbocation (an electrophile).
- For example, when methyl chloride (CH3Cl) reacts with AlCl3, it forms a methyl cation (CH3+) and AlCl4-.
- The generated carbocation (CH3+) then attacks the benzene ring, substituting one of the hydrogen atoms and forming an alkyl-substituted benzene (like toluene).
- The overall reaction results in the formation of an alkyl benzene and a hydrogen halide (HCl in the case of methyl chloride).
- Therefore, the reagent used for Friedel-Craft's reaction is anhydrous aluminum chloride (AlCl3).
The reagent used for Friedel-Craft's reaction is anhydrous AlCl3.
Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - Exercise-1 Part-3
2 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - Exercise-2 Part-1
11 Videos
- ORGANIC REACTION MECHANISMS - IIBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS - IIEXERCISE - APSP Part - 3
22 Videos
- MOLE CONCEPTBOOK - RESONANCE ENGLISHCHAPTER - MOLE CONCEPTEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
27 Videos
- ORGANIC REACTION MECHANISMS-IVBOOK - RESONANCE ENGLISHCHAPTER - ORGANIC REACTION MECHANISMS-IVEXERCISE - APSP PART-3
22 Videos
Similar Questions
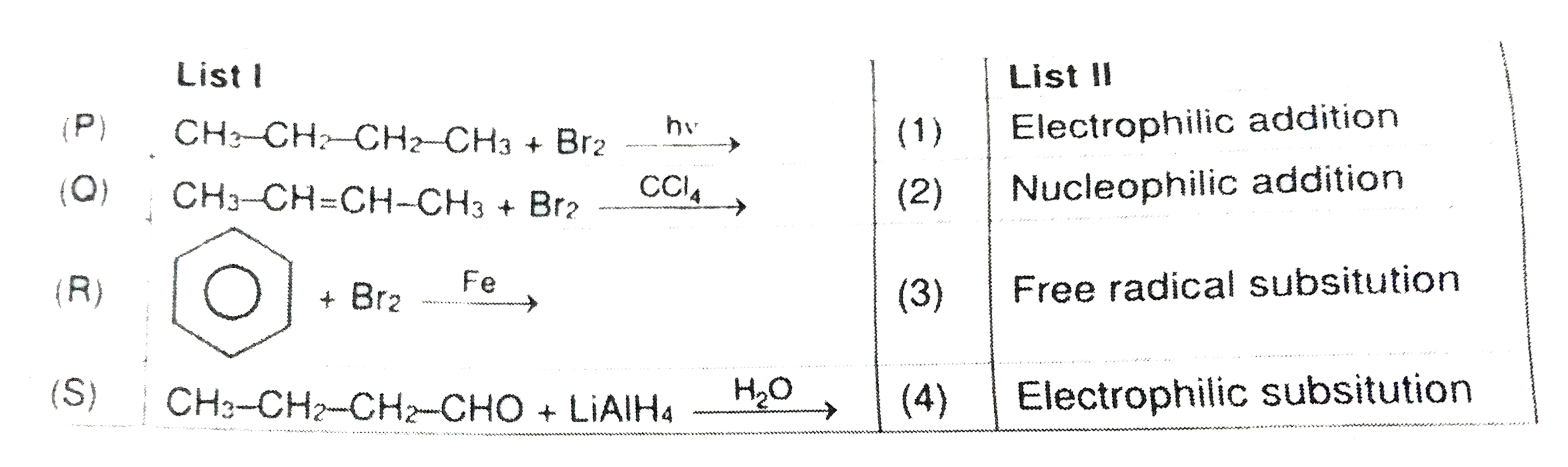
Match List I (Reaction ) with List II (Type of reaction ) and select the correct answer using the code given below the lists :
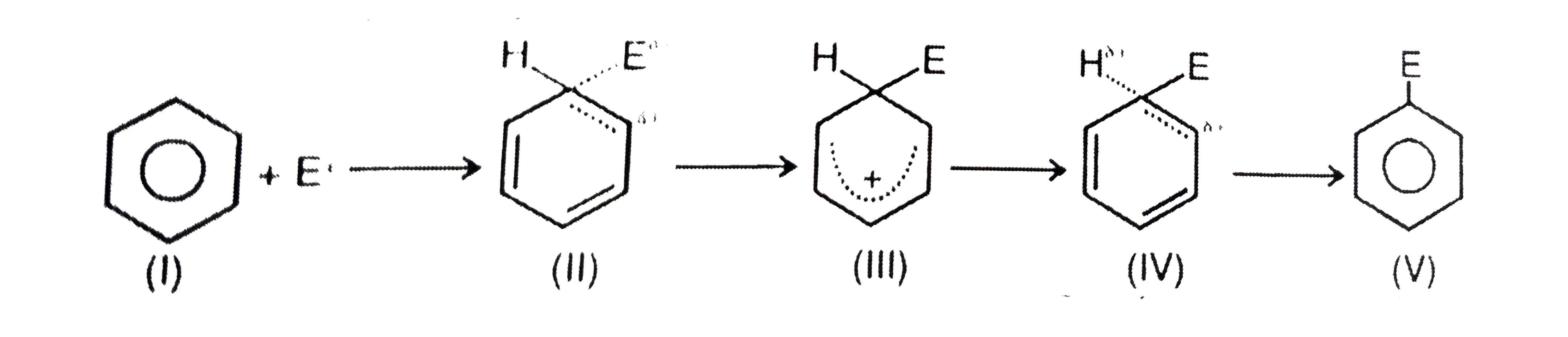
View SolutionWhich of the following species is expected to have maximum enthalpy in an electrophilic aromatic substitution reaction ?
View Solution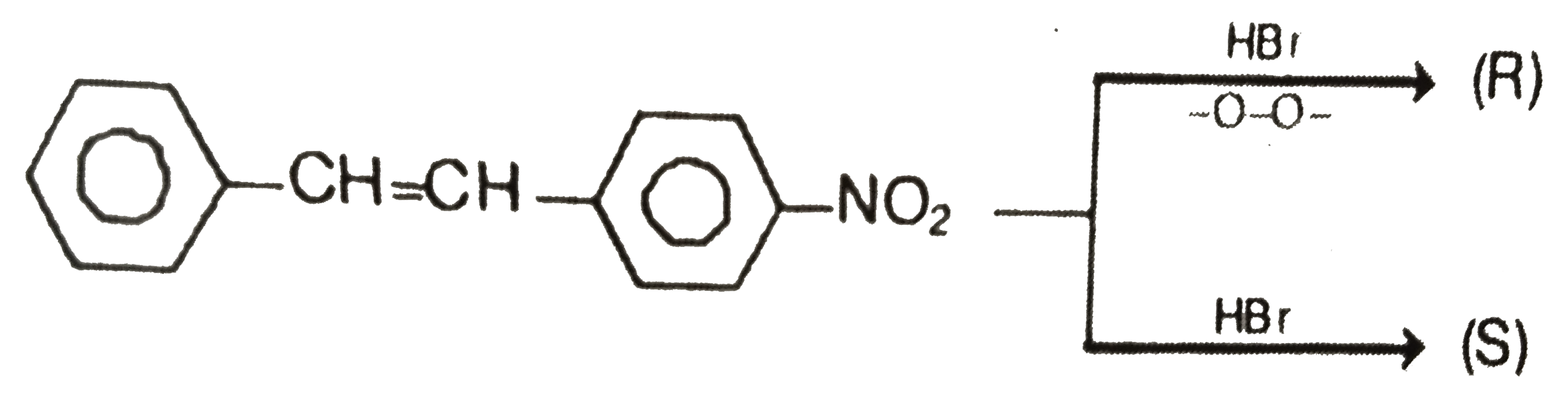
R & S are :
View SolutionA partially dried clay mineral contains 8%d water. The original sample contained 12% water and 45% sillica.
The % if sillica in the partially dried sample is nearly:
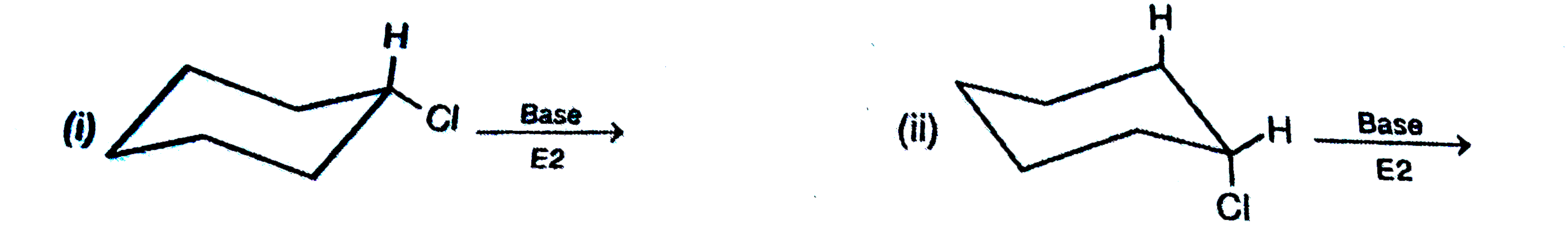
View SolutionRate of Bimolecular elimination (E2) reaction for the following :
View SolutionWhich of the following is Friedel - Craft's reaction
View SolutionName the reagent used in the Friedel Craft's alkylation of anisole.
View SolutionThe reagent used for Friedel-Craft's reaction is :
View SolutionAnhydrous AlCl3 is used as a catalyst in the friedel-crafts reaction because it is
View SolutionWhich of the given species cannot be used in Friedel Craft's reactions ?
View SolutionWhich of the following is not used in Friedel-Craft's reaction
View SolutionWhich of the following acids is used Friedel-Craft's reaction-
View SolutionWhich of the given species cannot be used in Friedel Craft's reactions ?
View SolutionAnhydrous AlCl_3 is used in the Friedel craft's reaction because it is
View SolutionThe function of AlCl3 in Friedel-Craft's reaction is:
View Solution

