An alkyl chloride produces a single alkene on reaction with sodium ethoxide and ethanol. The alkene further undergoes hydrogenation to yield 2- methylbutane. Identify the alkyl chloride from amongst the following
ClCH2C(CH3)2CH3
ClCH2CH2CH2CH3
ClCH2CH(CH3)CH2CH3
CH3C(Cl)(CH3)CH2CH3
The correct Answer is:C
C∣ClH2−C∣CH3H−CH2−CH3C2H5O−Na+−−−−−−−−→E2CH2=C∣CH3−CH2−CH3H2/Ni−−−→CH3−C∣CH3H−CH2−CH3
Topper's Solved these Questions
- ORGANIC REACTION MECHANISMS-IVBook:RESONANCE ENGLISHChapter:ORGANIC REACTION MECHANISMS-IVExercise:APSP PART-2
Explore 17 Videos
- ORGANIC REACTION MECHANISMS-IVBook:RESONANCE ENGLISHChapter:ORGANIC REACTION MECHANISMS-IVExercise:APSP PART-3
Explore 22 Videos
- ORGANIC REACTION MECHANISMS-IVBook:RESONANCE ENGLISHChapter:ORGANIC REACTION MECHANISMS-IVExercise:Exercise-3 Part-2 JEE (MAIN ) ONLINE PROBLEMS
Explore 10 Videos
- ORGANIC REACTION MECHANISMS - IIBook:RESONANCE ENGLISHChapter:ORGANIC REACTION MECHANISMS - IIExercise:APSP Part - 3
Explore 22 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 24 Videos
Similar Questions
An alkyl bromide produces a single alkene when it reacts with sodium ethoxide and ethanol. This alkene undergoes hydrogenation and produces 2-methylbutane. What is the alkybromide ?
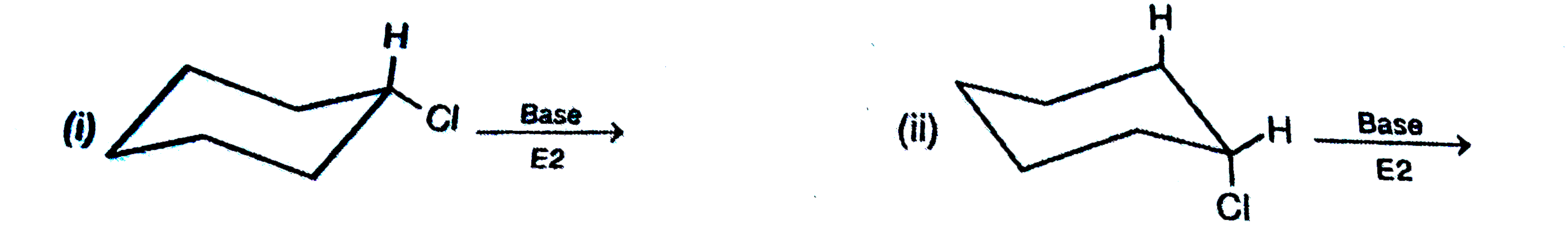
View SolutionRate of Bimolecular elimination (E2) reaction for the following :
View SolutionThe major product of the reaction is

View Solution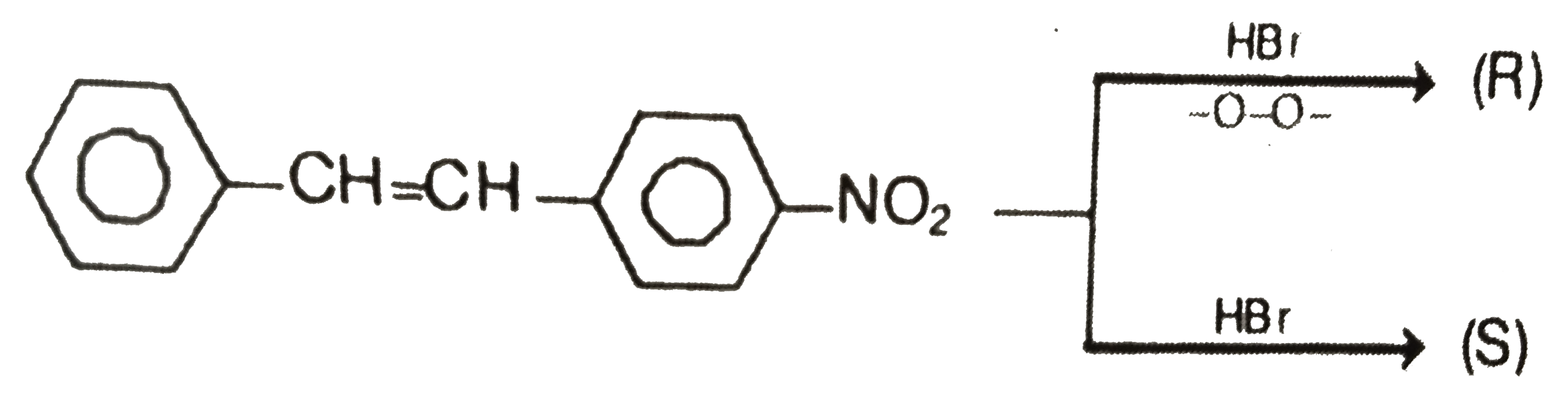
R & S are :
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionAn alkyl chloride produces a single alkene on reaction with sodium ethoxide and ethanol. The alkene further undergoes hydrogenation to yield 2-methylbutane. Identify the alkyl chloride from amongst the following :
View SolutionAn alkyl bromide produces a single alkene when it reacts with sodium ethoxide and ethanol. The alkene undergoes hydrogenation and produces 2-methylbutane. What is the identity of the alkyl bromide?
View SolutionAn alkyl bromide produces a single alkene when it reacts with sodium ethoxide and ethanol. This alkene undergoes hydrogenation and products 2-methylbutane. What is the identify of the alkybromide ?
View SolutionAn alkyl bromide produces a single alkene when it reacts with sodium ethoxide and ethanol. This alkene undergoes hydrogenation and produces 2-methyl butane. What is the identity of the alkyl bromide
View Solutionऐल्किल क्लोराइड की जलीय KOH से अभिक्रिया द्वारा एलकोहॉल बनता है लेलिन एलकोहॉलिक KOH की उपस्थिति में एल्कीन मुख्य उत्पाद के रूप में प्राप्त होती है। समझाइए।
View SolutionAn alkyl chloride produces a single alkene when it reacts with sodium ethoxide and ethanol . This alkene on hydrogenation produces 2-Methylbutane. What is the identity of the alkyl halide ?
View SolutionAn alkyl bromide produces a single alkene when it reacts with alcoholic KOH. This alkene on hydrogenation produces 2-methylbutane. Identify the alkyl halide
View SolutionAn alkyl bromide produces a single alkene when it reacts with sodium ethoxide and ethanol. This alkene on hydrogenation produces 2-methylbutane. What is the identify of the alkyl halide?
View SolutionPredict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene: (ii) 2-chloro-2-methylbutane.
View SolutionA single alkene is produced when an alkyl bromide reacts with sodium ethoxide and ethanol. This alkene undergeoes hydrogenation and produces 2 - methyl butane . What is the identity of the alkyl bromide ?
View SolutionPredict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene :
2-chloro -2- methylbutane
View Solution
