Complete the following reactions :
(i) CH3−CH2−C≡C−CH2−(CH2)6−CH2OH(i)Na/NH3(I)−−−−−−−−−−→(ii)H2O
(ii) 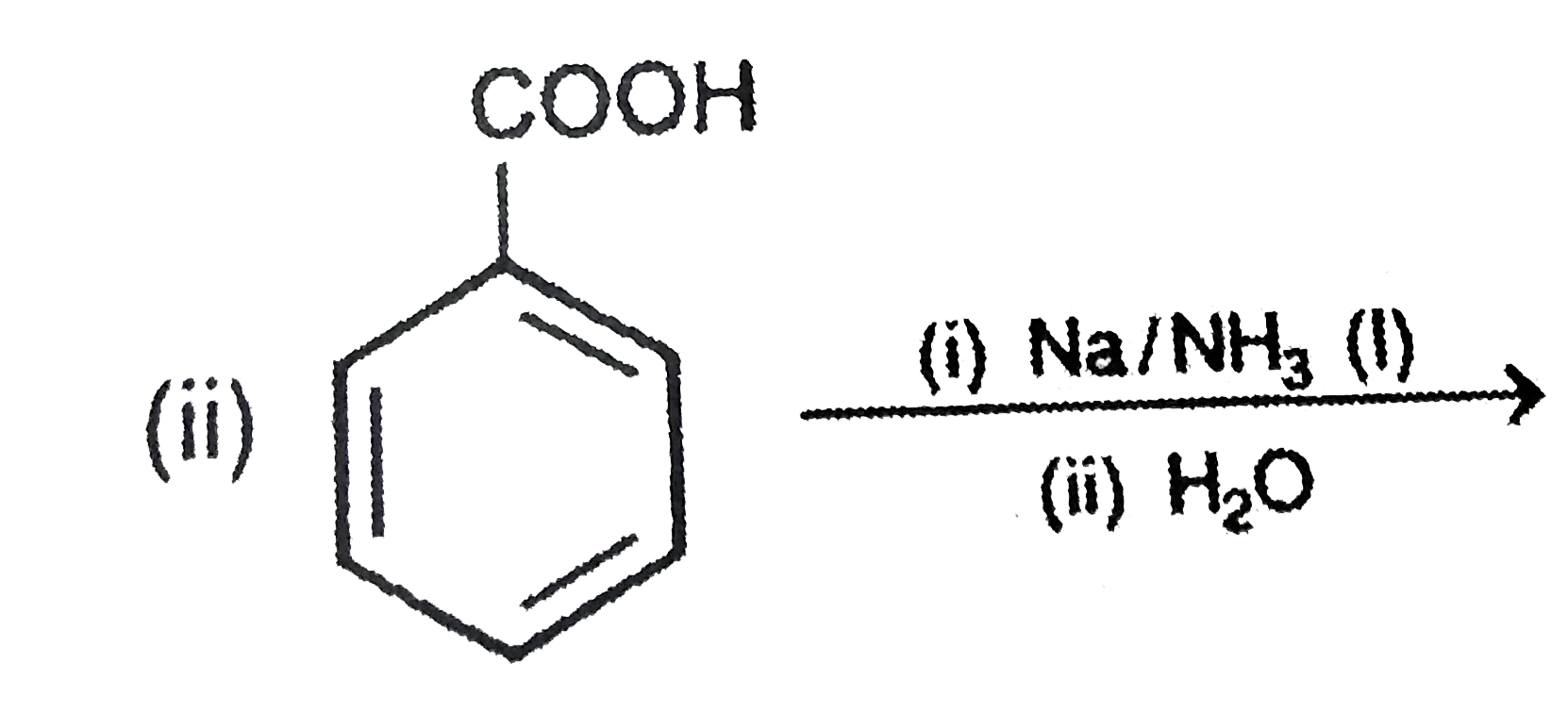
The correct Answer is:(i) CH3−CH2−CH=CHtrans−(CH2)7−CH2−OH (ii) (##RESCHMROHRE01003_A01##)
Step by step video solution for Complete the following reactions : (i) CH_(3)-CH_(2)-C-=C-CH_(2)-(CH_(2))_(6)-CH_(2)OH underset((ii) H_(2)O)overset((i) Na//NH_(3)(I))rarr (ii) by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-II Only one option correct type
Explore 26 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-III Match the column
Explore 2 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Comprehension Type
Explore 3 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 24 Videos
- S BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:S BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 4 Videos
Similar Questions
Name the gas liberated when AlH3 reacts with water.
View SolutionCH3COOH+NaOH→A+B
X+NaOH→C(gas)+D
C(gas) is:
View SolutionColour of ppt formed when HgCl2 reacts with KI is
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionS2: Plaster of paris is a hemihydrate of calcium sulphate obtained by heating the gypsum above 393K.
S2: Sodium carbonate is used in water softening.
S4: The order of mobilities of the alkali metal ions in aqueous solutions is Li+>Na+>K+>Rb+>Cs+
View SolutionClassify the following as primary, secondary and tertiary alcohols: (i) CH_(3)-overset(CH_(3))overset("| ")underset(CH_(3))underset("| ")(C )-CH_(2)OH (ii) H_(2)C=CH-CH_(2)OH (iii) CH_(3)-CH_(2)-CH_(2)-OH (iv)
View SolutionComplete the following reactions by identifying A,B and C.
(i) A+H2(g)Pb/BaSO4−−−−−−→(CH3)2CHCHO
(ii) CH3−CH3∣C∣CH3−C∣∣O−CH3+NaOI→B+C
View SolutionComplete the following reactions :
(i) CH3−CH2−C≡C−CH2−(CH2)6−CH2OH(i)Na/NH3(I)−−−−−−−−−−→(ii)H2O
(ii)
View SolutionWrite the main product (s) in each of the following reaction : (i) CH_(3) - underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-O-CH_(3)+HIrarr (ii) CH_(3) - CH = CH_(2) underset((ii)3H_(2)O_(2)//OH^(-))overset((i)B_(2)H_(6))rarr (iii) C_(6)H_(5) - OH underset((ii)CO_(2),H^(+))overset((i)aq.NaOH)rarr
View SolutionComplete the following reactions: (i) CH_(3)-CH_(2)-O-overset(CH_(3))overset("| ")underset(CH_(3))underset("| ")"C "-CH_(3)-HI overset("Heat")rarr (ii)
View SolutionComplete the following reaction:
(i) CH3−C∣∣O−CH3CH3,MgBr−−−−−−→Dry etherAH+−−→H2OB
(ii) CH3−CH2−CH2−CHOH2/Ni−−−→A
View SolutionClassify the following as primary, secondary and tertiary alcohols:
(i) CH3−CH3| C| CH3−CH2OH (ii) H2C=CH−CH2OH
(iii) CH3−CH2−CH2−OH
(iv)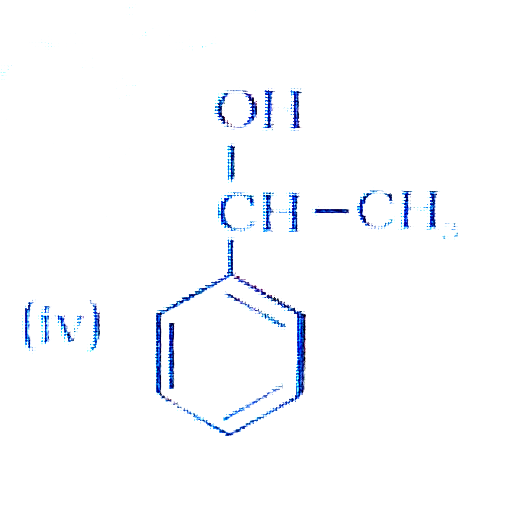
View SolutionClassify the following as primary alcohols, secondary alcohols and tertiary alcohols.
(i) CH3−CH3∣C∣CH3−CH2OH
(ii) H2C=CH−CH2OH
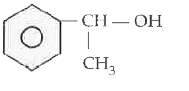
(iv) CH3−CH2−CH2−OH
View SolutionWrite the IUPAC name of the following
(i) CH3−CH3∣C∣CH3−CH2−CH2−CH2OH
(ii) CH3−NO2∣C∣I−CH2−CH2−COOH
View SolutionWrite the main product (s) in each of the following reaction : (i) CH_(3) - underset(CH_(3))underset(|)overset(CH_(3))overset(|)(C)-O-CH_(3)+HIrarr (ii) CH_(3) - CH = CH_(2) underset((ii)3H_(2)O_(2)//OH^(-))overset((i)B_(2)H_(6))rarr (iii) C_(6)H_(5) - OH underset((ii)CO_(2),H^(+))overset((i)aq.NaOH)rarr
View SolutionName the following compounds in the IUPAC: (i) CH_(3)-CH_(2)-overset(O)overset(||)C-OH (ii) CH_(3)-C-=C-underset(CH_(3))underset(|)CH_(2)
View Solution
