CH3CONH2H3O+−−−→ Product is :
The correct Answer is:CH3COOH+NH3
To determine the product of the reaction between acetamide (CH₃CONH₂) and hydronium ion (H₃O⁺), we can follow these steps:
Step 1: Identify the Reactants
The reactants in this reaction are acetamide (CH₃CONH₂) and hydronium ion (H₃O⁺). Acetamide is an amide, which contains a carbonyl group (C=O) bonded to a nitrogen atom (N).
Step 2: Understand the Reaction Type
The reaction involves an acid-base interaction where H₃O⁺ (the hydronium ion) acts as a strong acid. Acetamide can be protonated by the hydronium ion.
Step 3: Protonation of Acetamide
When acetamide is protonated by H₃O⁺, the nitrogen atom in the amide group gains a proton (H⁺), leading to the formation of an ammonium ion (CH₃CONH₃⁺).
Step 4: Hydrolysis of the Ammonium Ion
The protonated acetamide (CH₃CONH₃⁺) can undergo hydrolysis. The presence of water (from the hydronium ion) facilitates the cleavage of the C-N bond. This results in the formation of acetic acid (CH₃COOH) and ammonia (NH₃).
Step 5: Write the Products
The products of the reaction are:
1. Acetic acid (CH₃COOH)
2. Ammonia (NH₃)
Final Answer
Thus, the product of the reaction between acetamide and hydronium ion is acetic acid (CH₃COOH) along with the liberation of ammonia gas (NH₃).
---
The reactants in this reaction are acetamide (CH₃CONH₂) and hydronium ion (H₃O⁺). Acetamide is an amide, which contains a carbonyl group (C=O) bonded to a nitrogen atom (N).
The reaction involves an acid-base interaction where H₃O⁺ (the hydronium ion) acts as a strong acid. Acetamide can be protonated by the hydronium ion.
When acetamide is protonated by H₃O⁺, the nitrogen atom in the amide group gains a proton (H⁺), leading to the formation of an ammonium ion (CH₃CONH₃⁺).
The protonated acetamide (CH₃CONH₃⁺) can undergo hydrolysis. The presence of water (from the hydronium ion) facilitates the cleavage of the C-N bond. This results in the formation of acetic acid (CH₃COOH) and ammonia (NH₃).
The products of the reaction are:
1. Acetic acid (CH₃COOH)
2. Ammonia (NH₃)
Thus, the product of the reaction between acetamide and hydronium ion is acetic acid (CH₃COOH) along with the liberation of ammonia gas (NH₃).
Topper's Solved these Questions
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-II Only one option correct type
Explore 26 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-III Match the column
Explore 2 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Comprehension Type
Explore 3 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 24 Videos
- S BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:S BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 4 Videos
Similar Questions
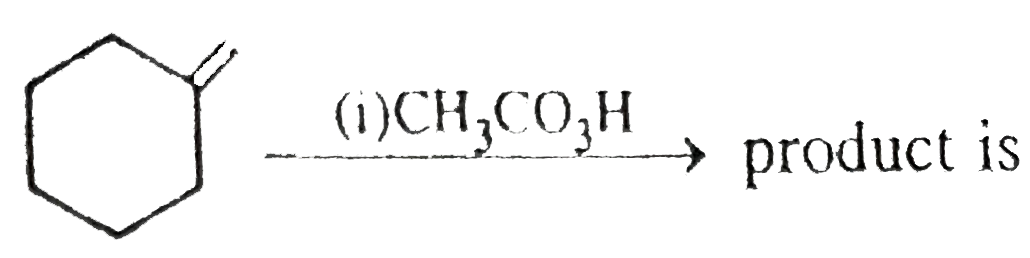
(i)CH3CO3H−−−−−−−−→ product is:-
View SolutionCH3OH+C2H5OHH⊕−−→ Products
View SolutionThe end product of the following reaction
CH3MgBr+CH2=CH−CHOH3O+−−−→ is :
View SolutionCH3−CH2CH3∣CHCH=CHCH3H3O+−−−→
find the product of the reaction .
View SolutionCH3−CH3∣CHCH=CHCH3H3O+−−−→
Major product of the reaction
View SolutionBut-1-ene(CH3COO)2Hg−−−−−−−−−→H3O?
The product in the above reaction is
View SolutionCH3CONH2H3O+−−−→ Product is :
View SolutionCH3−CH=CH2NOCl−−−→ Product, product is :
View SolutionCH3CH=CH2H/H2O−−−−→ major product is
View SolutionCH3CH2−O−CH3anhyHI−−−−→? Product is
View Solution
