Compound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives a acid (B), C8H6O4. (B) easily forms anhydride on heating. Identify the compound (A).
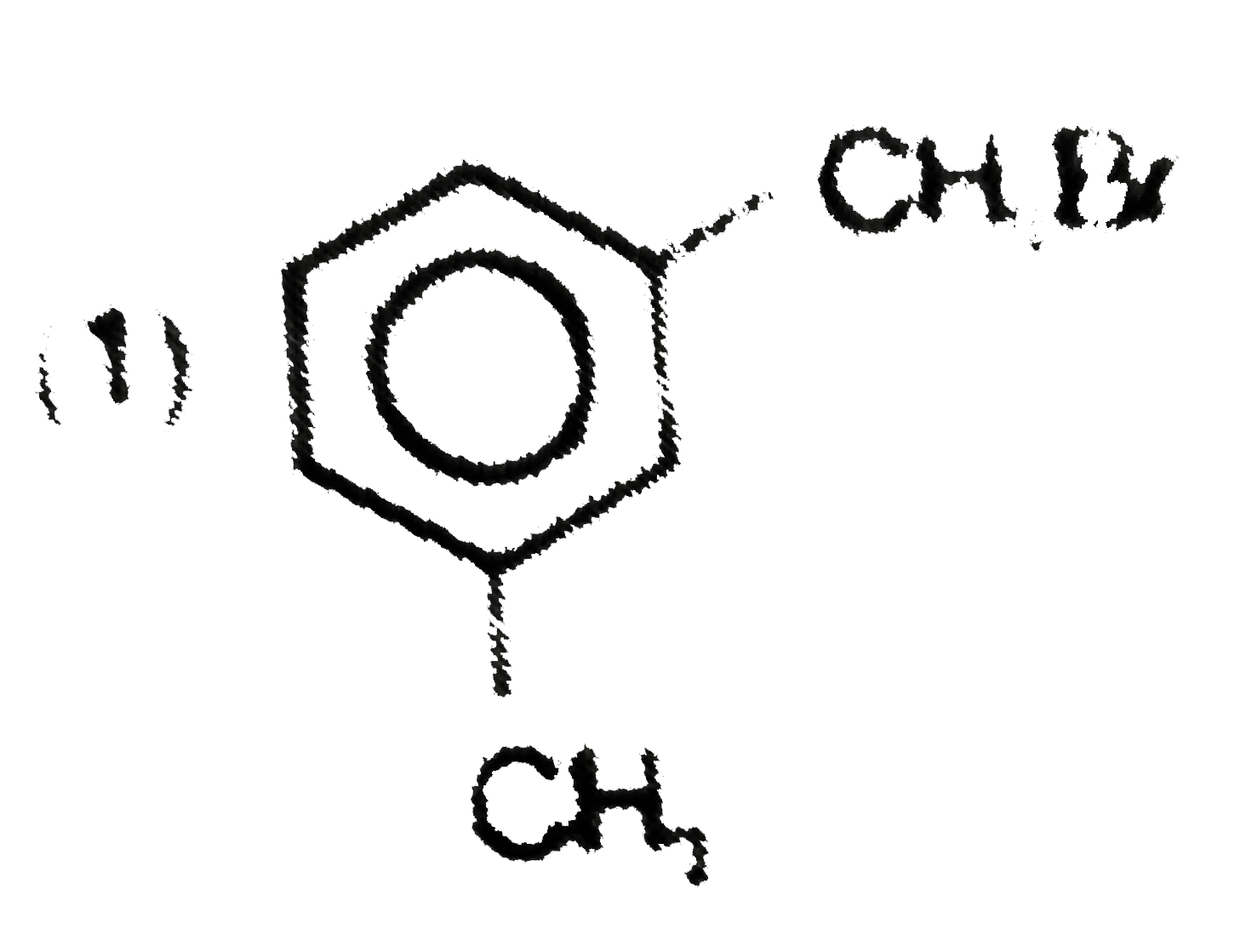
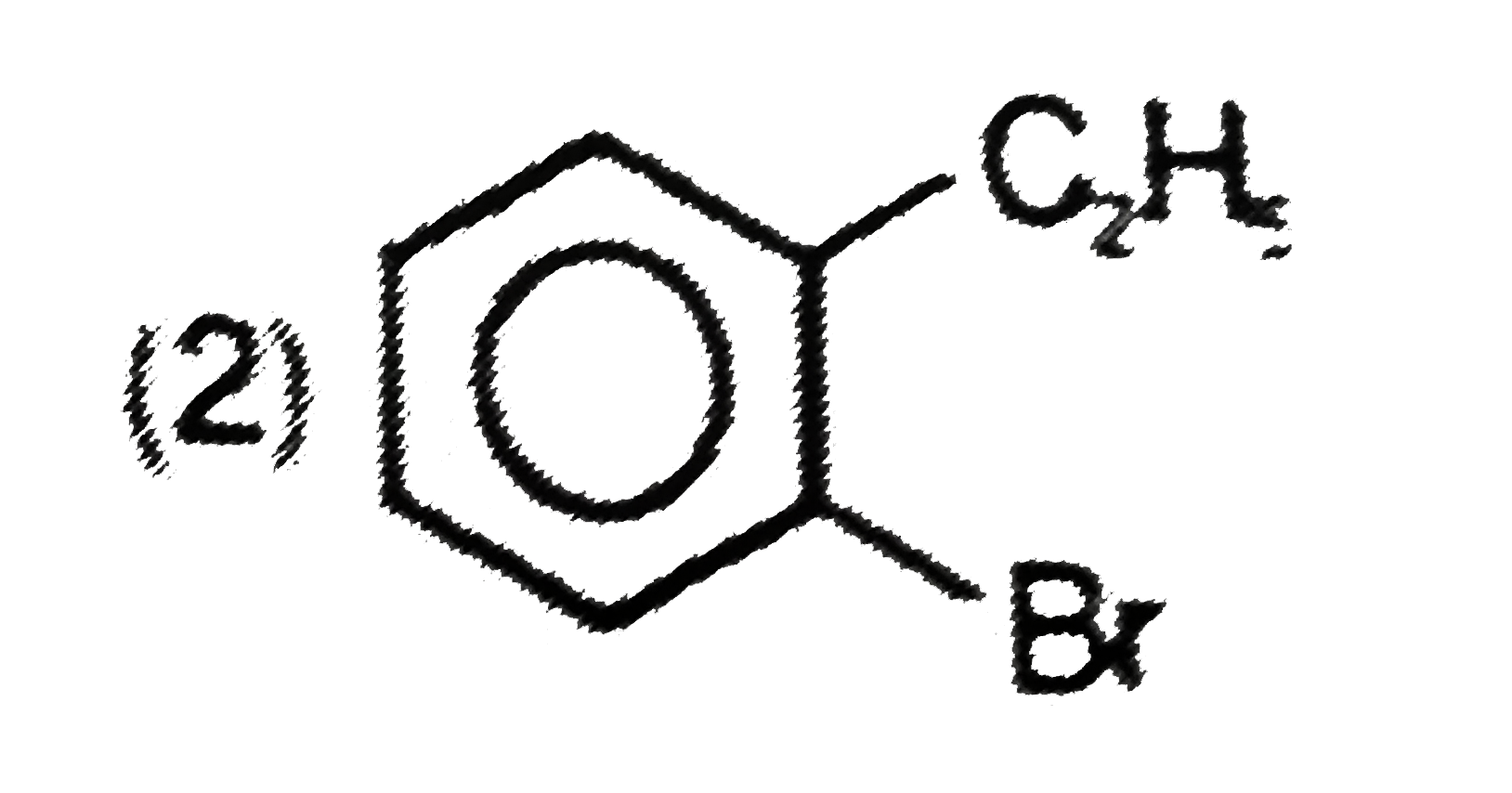
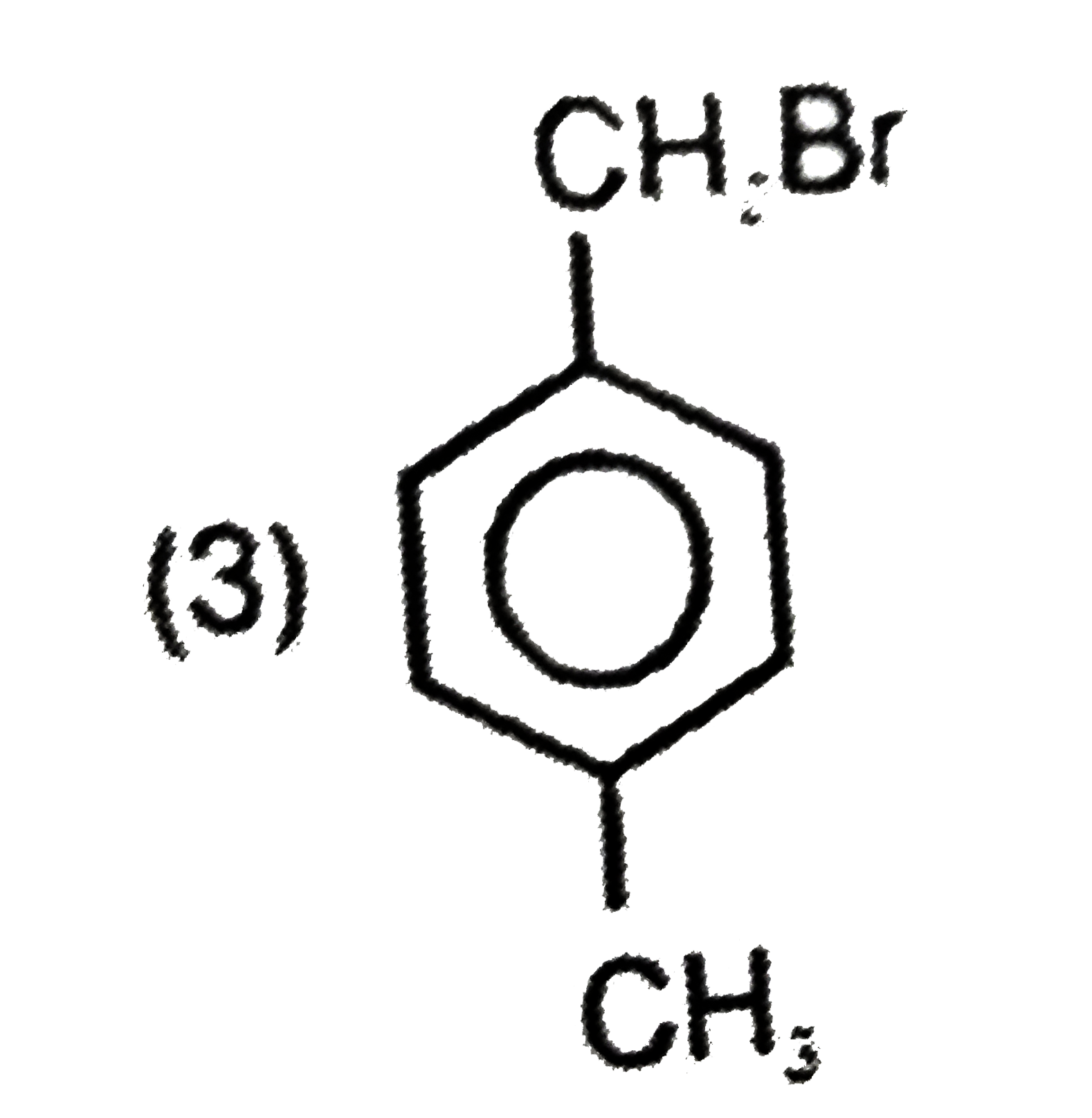
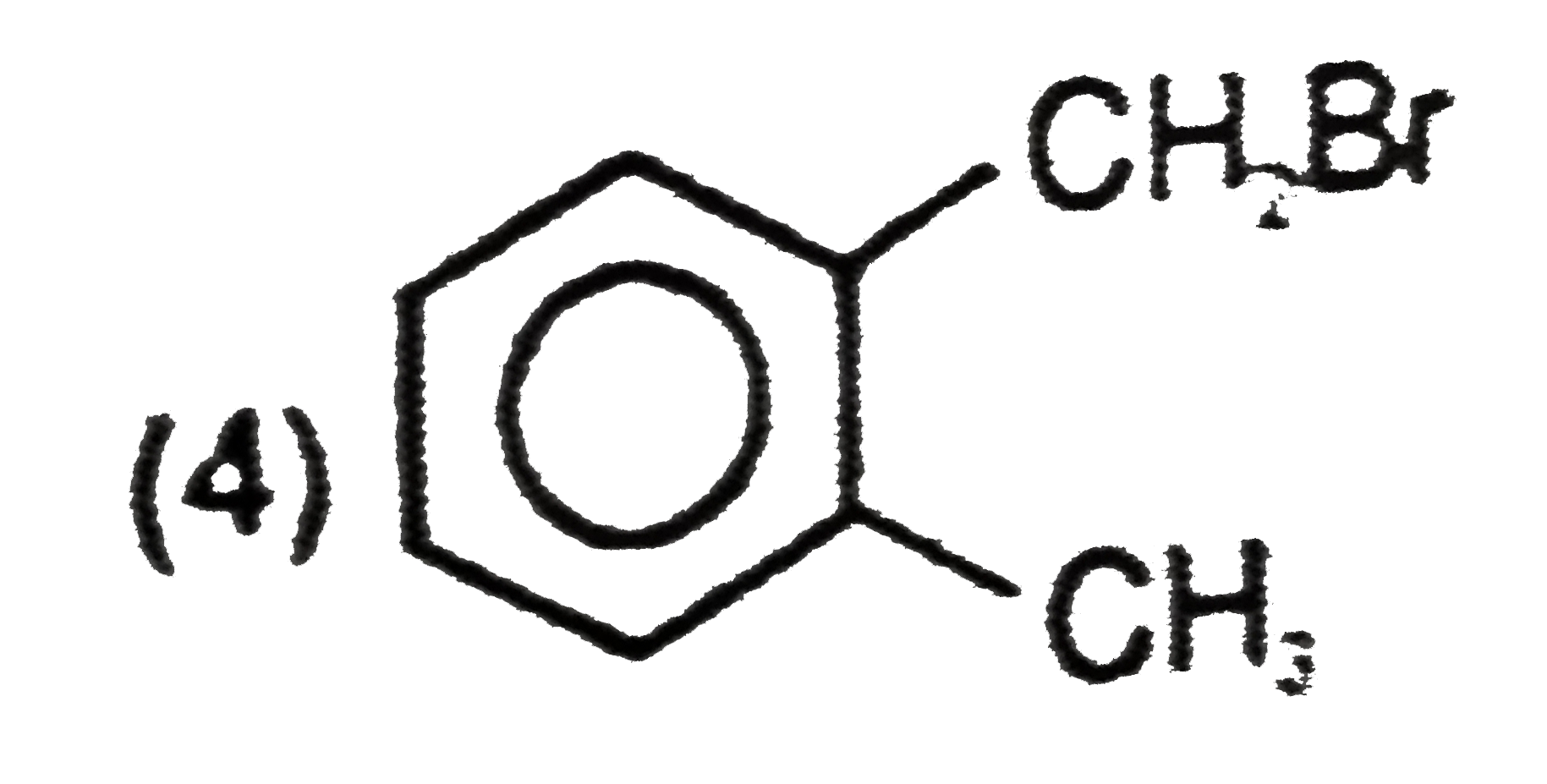
The correct Answer is:D
Step by step video solution for Compound (A), C_(8)H_(9)Br, gives a white precipitate when warmed with alcoholic AgNO_(3). Oxidation of (A) gives a acid (B), C_(8)H_(6)O_(4). (B) easily forms anhydride on heating. Identify the compound (A). by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBOOK - RESONANCE ENGLISHCHAPTER - REDUCTION, OXIDATION & HYDROLYSIS REACTIONSEXERCISE - JEE (MAIN) ONLINE PROBLEMS
13 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBOOK - RESONANCE ENGLISHCHAPTER - REDUCTION, OXIDATION & HYDROLYSIS REACTIONSEXERCISE - Additional problems for self Practice (Part-1)
30 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBOOK - RESONANCE ENGLISHCHAPTER - REDUCTION, OXIDATION & HYDROLYSIS REACTIONSEXERCISE - Part-I : Jee (Advanced) (Previous years)
12 Videos
- PERIODIC TABLE & PERIODICITYBOOK - RESONANCE ENGLISHCHAPTER - PERIODIC TABLE & PERIODICITYEXERCISE - ORGANIC CHEMISTRY(BASIC CONCEPTS)
24 Videos
- S BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - S BLOCK ELEMENTSEXERCISE - ORGANIC CHEMISTRY(Biomolecules & Polymer)
4 Videos
Similar Questions
The reagent needed for converting
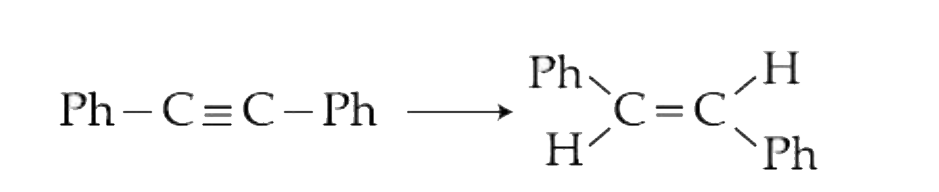 is :
is :
View SolutionPropyne and propene can be distinguished by ……….
View SolutionWhat would be the major product in the following reactions ?
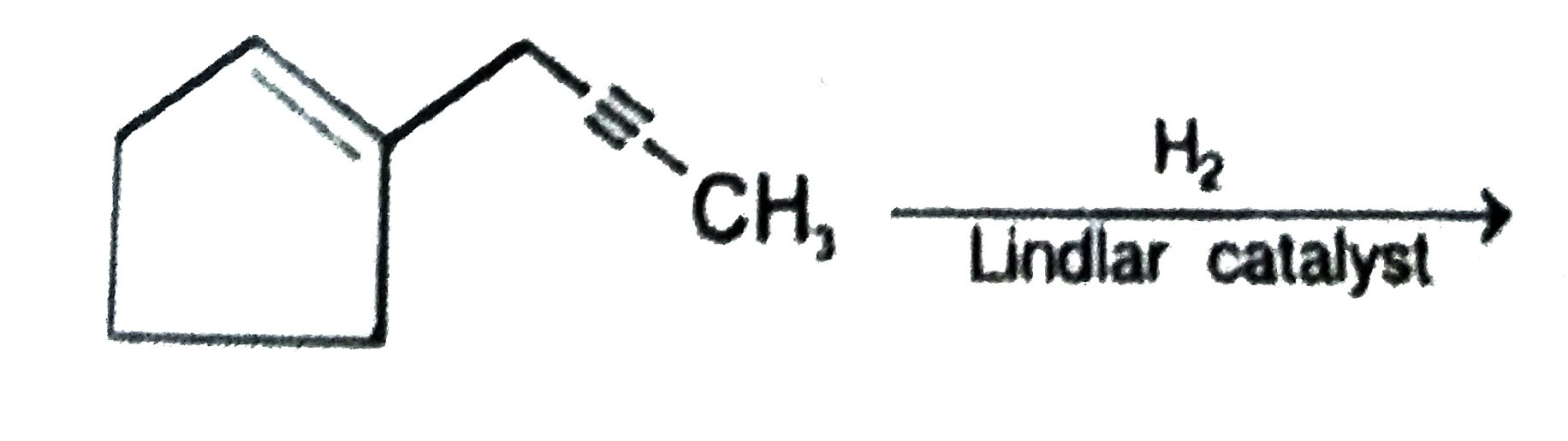
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionS2: Plaster of paris is a hemihydrate of calcium sulphate obtained by heating the gypsum above 393K.
S2: Sodium carbonate is used in water softening.
S4: The order of mobilities of the alkali metal ions in aqueous solutions is Li+>Na+>K+>Rb+>Cs+
View SolutionCompund (A)C_(8)H_(9) Br gives a white precipitate when warmed with alcoholic AgNO_(3) Oxidation of (A) gives an acid (B) C_(8) H_(6)O_(4) (B) easily anhydride on heating Identify the compound (A) .
View SolutionCompound (A) ,C8H9Br gives a white precipitate when warmed with alcoholic AgNO3 Oxidation of (A) gives an acid (B) C8H6O4 (B) easily forms anhydride on heating Identify the compound (A)
View SolutionCompound (A) C_(8)H_(9)Br , gives a light yellow precipitate when warmed with alcoholic AgNO_(3) (B) easily form anhydride on heating. Identify the compound (A)
View SolutionCompound (A) C_(8)H_(9)Br gives light yellow precipitate when warmed with alcoholic AgNO_(3) solution. Oxidation of (A) gives (B). C_(8)H_(6)O_(4) . The compound (B) easily forms an anhydride on heating. The compound (A) is:
View SolutionCompound (A) ,C8H9Br gives a white precipitate when warmed with alcoholic AgNO3 Oxidation of (A) gives an acid (B) C8H6O4 (B) easily forms anhydride on heating Identify the compound (A)
View SolutionCompound (A), C_(6)H_(3)Br , gives a white precipitate when warmed with alcoholic AgNO_(3) . Oxidation of (A) gives an acid (B). C_(6)H_(3)O_(4) . (B) easily forms anhydride on on heating. Identify the compound (A).
View SolutionCompound (A) C_(8)H_(9)Br , gives a white precipitate when warmed with alcoholic AgNO_(3) . Oxidation of (A) gives an acid (B), C_(8)H_(6)O_(4) . (B) easily forms anhydride on heating. Identify the compound (A)
View SolutionCompound (A) ,C8H9Br gives a white precipitate when warmed with alcoholic AgNO3 Oxidation of (A) gives an acid (B) C8H6O4 (B) easily forms anhydride on heating Identify the compound (A)
View SolutionCompound (A), C8H9Br, gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of (A) gives an acid (B) C8H6O4. (B) easily forms anhydribe on heating. Identify the compound (A).
View SolutionCompound (A), C_(8)H_(9)Br , gives a light yellow precipitate when warmed with alcoholic AgNO_(3) . Oxidation of (A) gives an acid (B), C_(8)H_(6)O_(4) . (B) easily forms anhydride on heating. Identify the compound (A).
View Solutionयौगिक (A) C8H9Br ऐल्कॉहॉलिक aGno3 के साथ गर्म करने पर एक सफेद अवक्षेप देता है। (A) के उपचयन से एक अम्ल (B) C8H6O4 प्राप्त होता है। (B) गर्म करने पर सरलता से एनहाइड्राइड बना देता है। यौगिक (A) है-
View Solution

