The trans-alkenes are formed by the reduction of alkynes with
Na/liq.NH3
Sn−HCl
H2−Pd/C,BaSO4
NaBH4
The correct Answer is:A
Step by step video solution for The trans-alkenes are formed by the reduction of alkynes with by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:JEE (MAIN) ONLINE PROBLEMS
Explore 13 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Additional problems for self Practice (Part-1)
Explore 30 Videos
- REDUCTION, OXIDATION & HYDROLYSIS REACTIONSBook:RESONANCE ENGLISHChapter:REDUCTION, OXIDATION & HYDROLYSIS REACTIONSExercise:Part-I : Jee (Advanced) (Previous years)
Explore 12 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:ORGANIC CHEMISTRY(BASIC CONCEPTS)
Explore 24 Videos
- S BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:S BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 4 Videos
Similar Questions
The reagent needed for converting
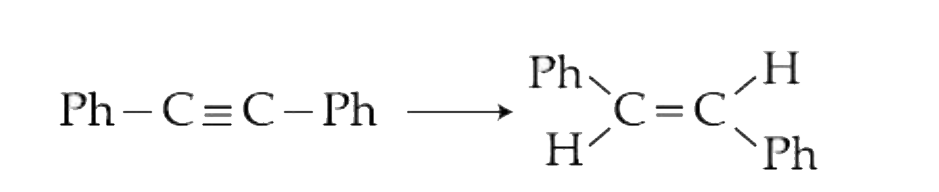 is :
is :
View SolutionPropyne and propene can be distinguished by ……….
View SolutionWhat would be the major product in the following reactions ?
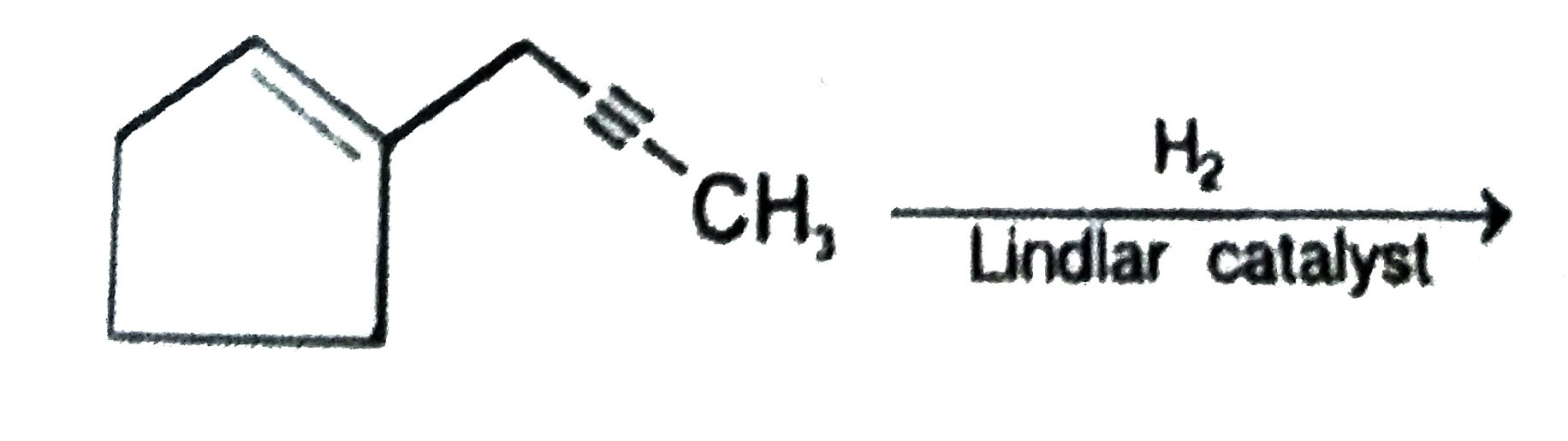
View SolutionAn element with atomic number 107 has recently been discovered. Its block, group number, period and outshell electronic configuration respectively are :
View SolutionS2: Plaster of paris is a hemihydrate of calcium sulphate obtained by heating the gypsum above 393K.
S2: Sodium carbonate is used in water softening.
S4: The order of mobilities of the alkali metal ions in aqueous solutions is Li+>Na+>K+>Rb+>Cs+
View SolutionAlkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of 2-butyne show the geometrical isomerism ?
View SolutionReduction of an alkyne to the double bond stage can yield a cis alkene or trans alkene (except in cases where triple bond is at the end of the chain ) Reduction of alknes with sodium or lithium in liquid NH_(3) yields predominantly trans alkene. Hydrogenation of alkynes with Lindlar's catalyst or a nickel boride called P_(2) catalyst yeilds is alkene (as high as 98%) If Pd/C/ H_(2) is used in the absence of a catalyst poison, two equivlanet of H_(2) are added forming alkanes. Reduction of alkynes to trans alkene by Li//NH_(3) is carried out in the presence of dry ice. This is because of
View SolutionThe trans-alkenes are formed by the reduction of alkynes with :
View SolutionThe trans-alkenes are formed by the reduction of alkynes with
View SolutionThe trans-alkenes are formed by the reduction of alkynes with
View SolutionAlkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of 2-butyne show the geometrical isomerism ?
View SolutionThe trans-alkenes are formed by the reduction of alkynes with :
View SolutionThe trans-alkenes are formed by the reduction of alkynes with-
View SolutionAlkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of but-2-yne show the geometrical isomerism?
View SolutionThe trans-alkenes are formed by the reduction of alkynes with
View SolutionAlkynes on reduction with sodium in liquid ammonia form trans alkenes. Will the butene thus formed on reduction of 2-butyne show the geometrical isomerism ?
View Solution
