In hexagonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them. A space-filling model of this structure, called hexagonal close-packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Each one of these three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be ‘r’.
The volume of this HCP unit cell is
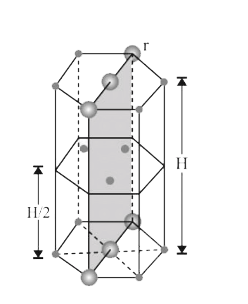
24√2r3
16√2r3
12√2r3
643√3r3
The correct Answer is:A
Step by step video solution for In hexagonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them. A space-filling model of this structure, called hexagonal close-packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Each one of these three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be ‘r’. The volume of this HCP unit cell is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLID STATEBook:RESONANCE ENGLISHChapter:SOLID STATEExercise:Exercise -3 (Part-II)
Explore 18 Videos
- SOLID STATEBook:RESONANCE ENGLISHChapter:SOLID STATEExercise:Exercise -3 (JEE -MAIN)
Explore 12 Videos
- SOLID STATEBook:RESONANCE ENGLISHChapter:SOLID STATEExercise:Exercise -2 (Part-IV Comprehension)
Explore 8 Videos
- S BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:S BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Biomolecules & Polymer)
Explore 4 Videos
- STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYBook:RESONANCE ENGLISHChapter:STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYExercise:Section -B
Explore 18 Videos
Similar Questions
Na and Mg crystallize in bcc- and fcc-type crystals, respectively, then the number of atoms of Na and Mg present in the unit cell of their respective crystal is
View SolutionIn a face centered cubic lattice atoms A are at the corner points and atoms B at the face centered points. If atoms B is missing from one of the face centered points, the formula of the ionic compound is
View SolutionConsider the figure given for solid XY. Answer the following questions :
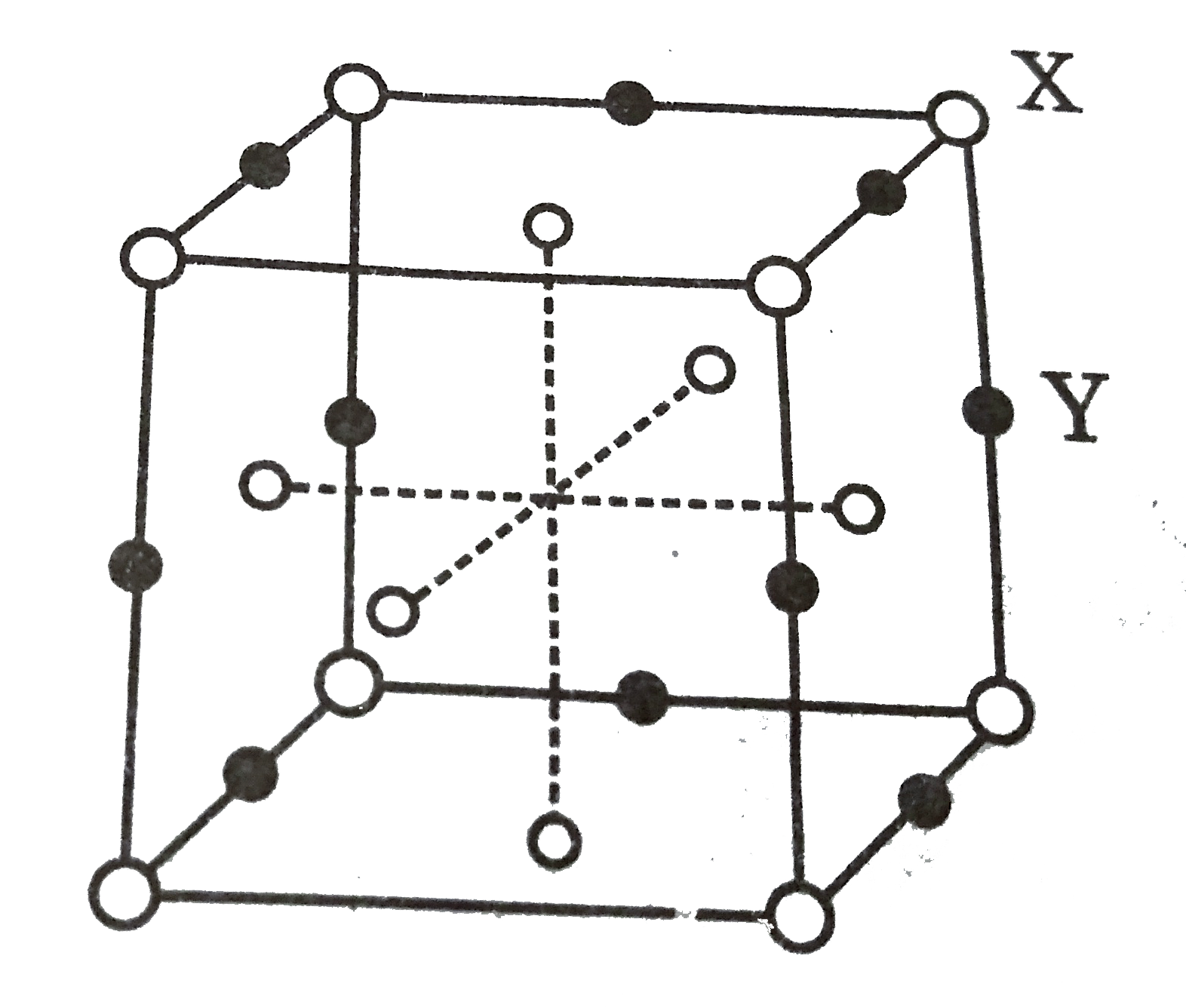
The number of XY units per unit cell is:
View SolutionS2: Plaster of paris is a hemihydrate of calcium sulphate obtained by heating the gypsum above 393K.
S2: Sodium carbonate is used in water softening.
S4: The order of mobilities of the alkali metal ions in aqueous solutions is Li+>Na+>K+>Rb+>Cs+
View SolutionIn Kjeldahl's method, nitrogen present is estimated as
View SolutionIn hexagonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them. A space-filling model of this structure, called hexagonal close-packed (hcp), is constitued of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Each one of these three spheres touched three spheres of the bottom layer. Finally, the second layer is convered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be 'r'.
The volume of this hcp unit cell is
View SolutionIn hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them. A space -filling model of this structure, called hexagonal close-packed (HCP), is consituted of a sphere on a flat surface surrouneded in the same plane by six identical spheres as closely possible. Three sphere are then palces over the first layer so that they touch each other and represent second layer is covered with third layer that is identical to the bottom layer in relative position. Assume radius of enery sphere to be r.
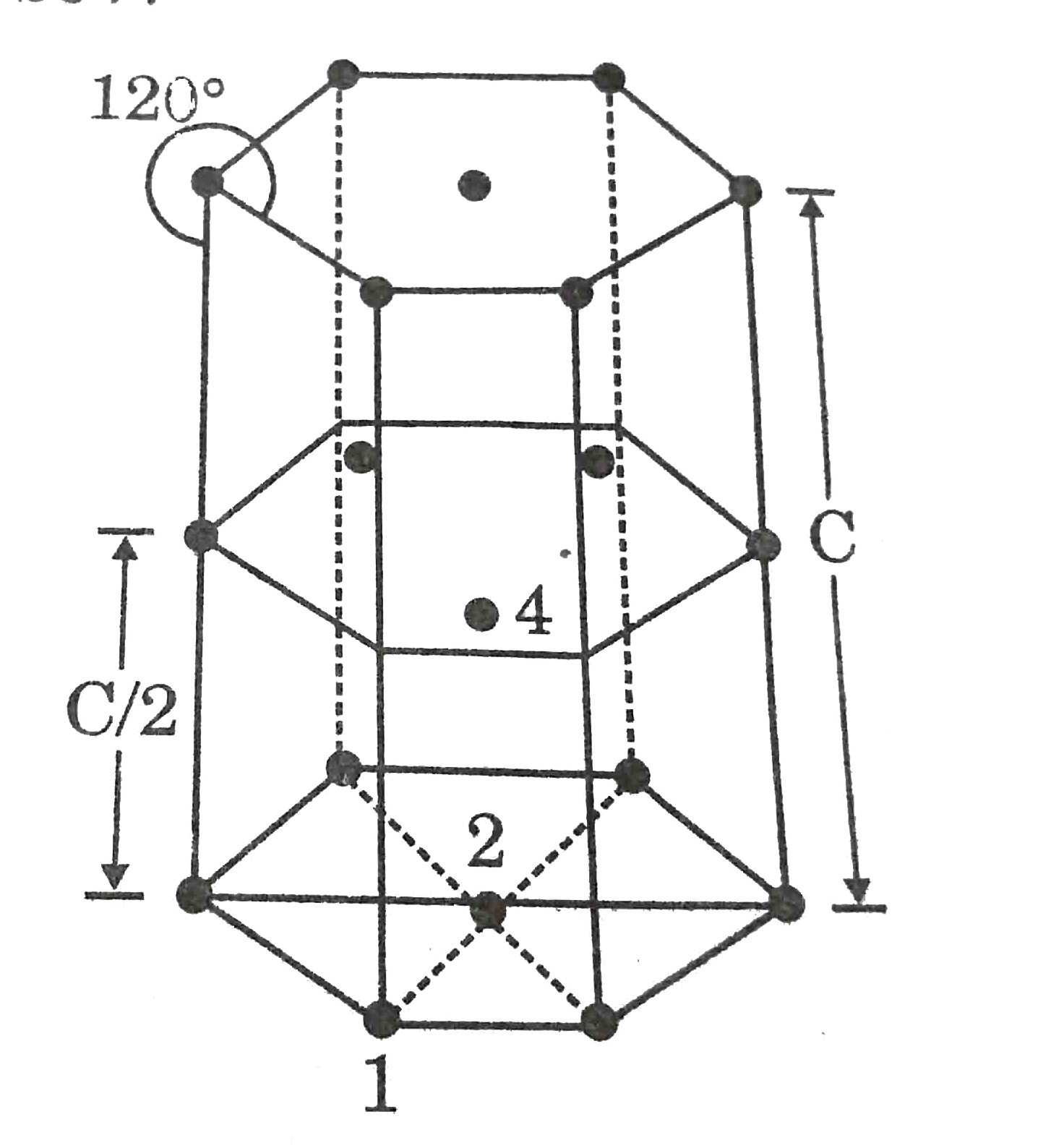
The number of atoms in the HPC unit cell is :
View SolutionIn a hexaonal system system of cycstals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are refular hexagons, and three atoms are sandwiched in between them. A space-cilling model of this structure, called hexagonal close-paked is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spherres are then placed overt the first layer so that they toych each other and represent the second layer so that they toych each other and present the second layer. Each one of the three spheres touches three spheres of the bottom layer. Finally, the second layer is convered with a third layer identical to the bottom layer in relative position. Assume the radius of every sphere to be r.
The voume of this hcp unit cell is
View SolutionIn hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagon and three atoms are sandwiched in between them. A space filling model of this structure, called hexagonal close packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical sphere as closely as possible. Three spheres are then placed over the first layer so that they touch other and represent the second layer. Each one of the three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be same
The number of atoms in this HCP unit cell is
View SolutionIn hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagon and three atoms are sandwiched in between them. A space filling model of this structure, called hexagonal close packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical sphere as closely as possible. Three spheres are then placed over the first layer so that they touch other and represent the second layer. Each one of the three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be same
The volume of this HCP unit cell is
View SolutionIn hexagonal system of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexagon and three atoms are sandwiched in between them. A space filling model of this structure, called hexagonal close packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical sphere as closely as possible. Three spheres are then placed over the first layer so that they touch other and represent the second layer. Each one of the three spheres touches three spheres of the bottom layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be same
The empty space in this HCP unit cell is
View SolutionIn hexagonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism.Here, the top and bottom of the cell are regular hexagons and three atoms are sandwiched in between them.A space-fitting model of this structure, called hexagonal closed-packed (HCP), is constitution of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible Three spheres are then placed over the first layer so that they touch each other and represent the second layer.Each one of these three spheres touches three spheres of the bottom layer.Finally, the second layer is covered with third layer that is identical to the bottom layer in relative position.Assume radius of even sphere to be 'r'.
The volume of this HCP unit cell is :
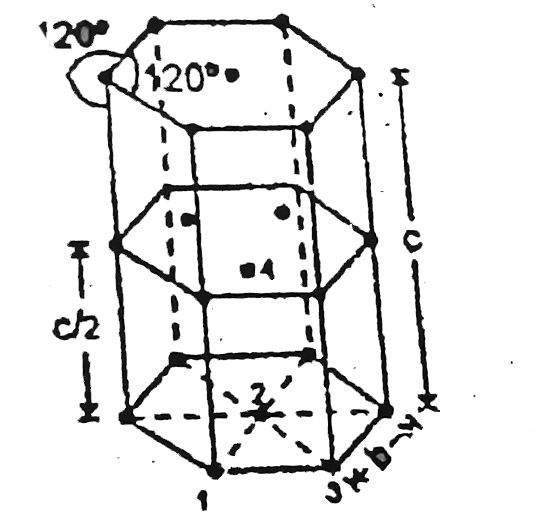
View SolutionIn hexagonal systems of crystals, a frequently ecounted arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are reqular hexagons and three atoms are sandwiched in between them. A space-filling model of this structure called hexagonal close-packed (HCP), is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. There spheres are then placed over the first layer so that they touch each other and represent the second layer. Each one of these three sphere touches three spheres of the bottom layer. Finally the second layer is covered with third layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be 'r'

The volume of this HCP unit cell is
View SolutionIn hexogonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexongonas and three atoms are sandwiched inbetween them. A space filling model of this structure called haxagonal closed packed (HCP) is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible . Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be 'r'. The number of atoms in this HCP unit cell is
View SolutionIn a hexaonal system system of cycstals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are refular hexagons, and three atoms are sandwiched in between them. A space-cilling model of this structure, called hexagonal close-paked is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible. Three spherres are then placed overt the first layer so that they toych each other and represent the second layer so that they toych each other and present the second layer. Each one of the three spheres touches three spheres of the bottom layer. Finally, the second layer is convered with a third layer identical to the bottom layer in relative position. Assume the radius of every sphere to be r.
The voume of this hcp unit cell is
View SolutionIn hexogonal systems of crystals, a frequently encountered arrangement of atoms is described as a hexagonal prism. Here, the top and bottom of the cell are regular hexongonas and three atoms are sandwiched inbetween them. A space filling model of this structure called haxagonal closed packed (HCP) is constituted of a sphere on a flat surface surrounded in the same plane by six identical spheres as closely as possible . Three spheres are then placed over the first layer so that they touch each other and represent the second layer. Finally, the second layer is covered with a third layer that is identical to the bottom layer that is identical to the bottom layer in relative position. Assume radius of every sphere to be 'r'. The empty space in this HCP unit cell is
View Solution
