A crystalline solid of a pure substance has a face – centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8 g cm−3, then the number of atoms present in 256 g of the crystal is N×1024 . The value of N is ____________.
The correct Answer is:2
Step by step video solution for A crystalline solid of a pure substance has a face – centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8 g cm^(-3), then the number of atoms present in 256 g of the crystal is N xx 10^(24) . The value of N is ____________. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SOLID STATEBOOK - RESONANCE ENGLISHCHAPTER - SOLID STATEEXERCISE - Exercise -3 (Part-II)
18 Videos
- SOLID STATEBOOK - RESONANCE ENGLISHCHAPTER - SOLID STATEEXERCISE - Exercise -3 (JEE -MAIN)
12 Videos
- SOLID STATEBOOK - RESONANCE ENGLISHCHAPTER - SOLID STATEEXERCISE - Exercise -2 (Part-IV Comprehension)
8 Videos
- S BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - S BLOCK ELEMENTSEXERCISE - ORGANIC CHEMISTRY(Biomolecules & Polymer)
4 Videos
- STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYBOOK - RESONANCE ENGLISHCHAPTER - STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRYEXERCISE - Section -B
18 Videos
Similar Questions
Na and Mg crystallize in bcc- and fcc-type crystals, respectively, then the number of atoms of Na and Mg present in the unit cell of their respective crystal is
View SolutionIn a face centered cubic lattice atoms A are at the corner points and atoms B at the face centered points. If atoms B is missing from one of the face centered points, the formula of the ionic compound is
View SolutionConsider the figure given for solid XY. Answer the following questions :
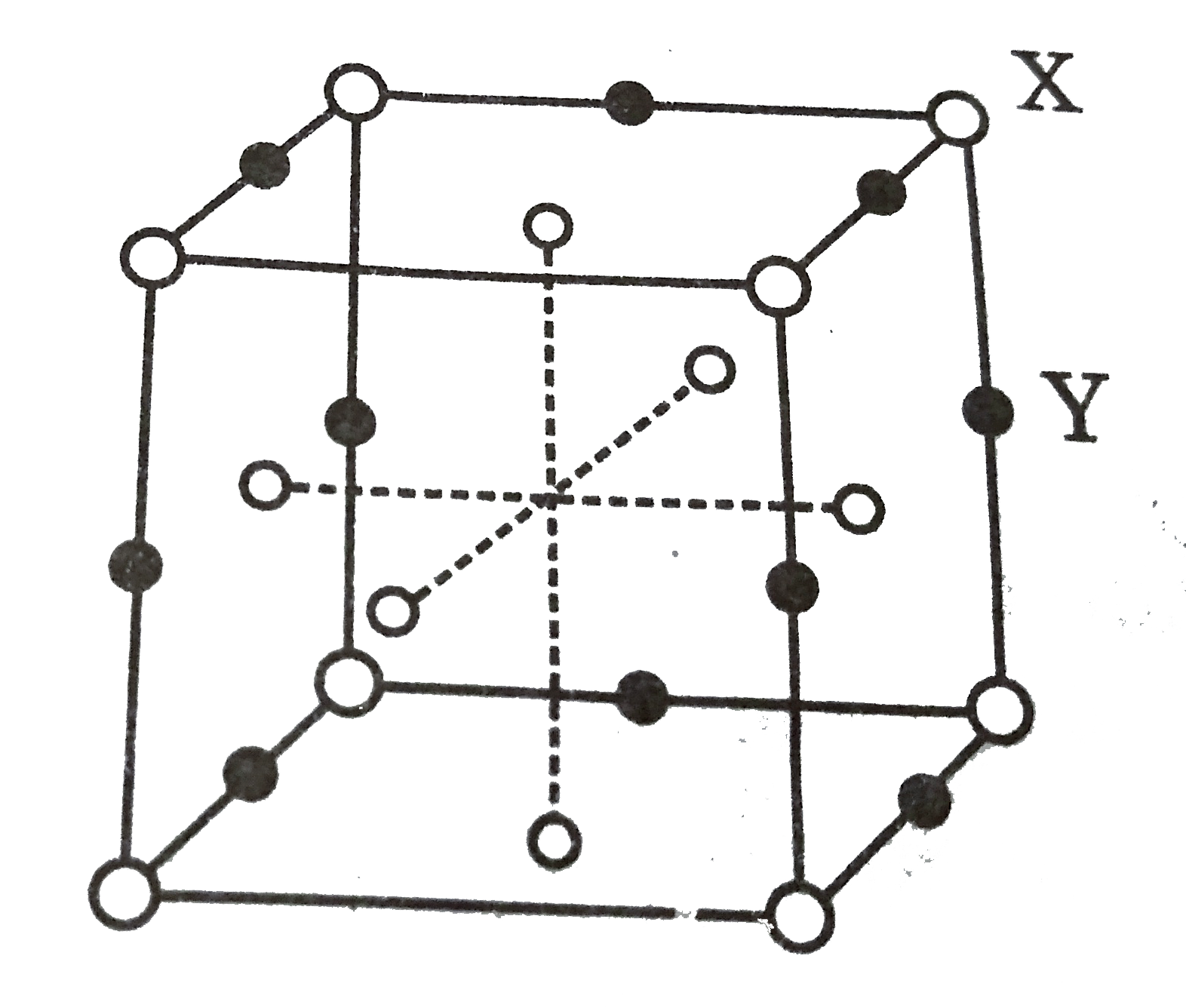
The number of XY units per unit cell is:
View SolutionS2: Plaster of paris is a hemihydrate of calcium sulphate obtained by heating the gypsum above 393K.
S2: Sodium carbonate is used in water softening.
S4: The order of mobilities of the alkali metal ions in aqueous solutions is Li+>Na+>K+>Rb+>Cs+
View SolutionIn Kjeldahl's method, nitrogen present is estimated as
View SolutionA crystalline solid of a pure substance has a face-centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8gcm−3, then the number of atoms present in 256g of the crystal is N×1024. The value of N is
View SolutionA substance has a face centred cubic crystal with a density 1.984 g cm−3 and edge length 630 pm. Calculate the molar mass of the substance
View SolutionA substance forms face-centred cubic crystals. Its density is 1.984 g cm^(-3) and the edge length of the unit cell is 630 pm. Calculate the molar mass of the substance.
View SolutionA crystalline solid of a pure substance has a face-centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8gcm−3, then the number of atoms present in 256g of the crystal is N×1024. The value of N is
View SolutionA crystalline solid of a pure substance has a face-centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8gcm−3, then the number of atoms present in 256g of the crystal is N×1024. The value of N is
View SolutionCalculate the number of atoms present in 2 grams of crystal which has face- centred cubic (FCC) crystal lattice having edge length of 100 pm and density 10 gcm−3
View SolutionA substance which has face-centred cubic crystal has a density of 2.16 g cm^(-3) and the edge lenghtof the unit cell equal to 564 pm. The molar mass of the substanceis
View SolutionA crystalline solid of a pure substance has a face-centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8gcm−3, then the number of atoms present in 256g of the crystal is N×1024. The value of N is
View SolutionA crystalline solid of pure substance has a face - centred cubic structure with a cell edge of 400 pm. If the density of the substance in the crystal is 8 g cm−3, then the number of atoms present in 128 g of the crystal is
View Solutionएक शुद्ध पदार्थ के एक क्रिस्टलीय ठोस की फलक - केन्द्रित धन संरचना के साथ कोष्ठिका कोर की लम्बाई 400 pm है | यदि क्रिस्टल के पदार्थ का घनत्व 8gcm−3 है, तो क्रिस्टल के 256g में उपस्थित परमाणुओं की कुल संख्या N×1024 है | N का मान है
View Solutionएक शुद्ध पदार्थ के एक क्रिस्टलीय ठोस की फलक - केन्द्रित धन संरचना के साथ कोष्ठिका कोर की लम्बाई 400 pm है | यदि क्रिस्टल के पदार्थ का घनत्व 8gcm−3 है, तो क्रिस्टल के 256g में उपस्थित परमाणुओं की कुल संख्या N×1024 है | N का मान है
View Solution

