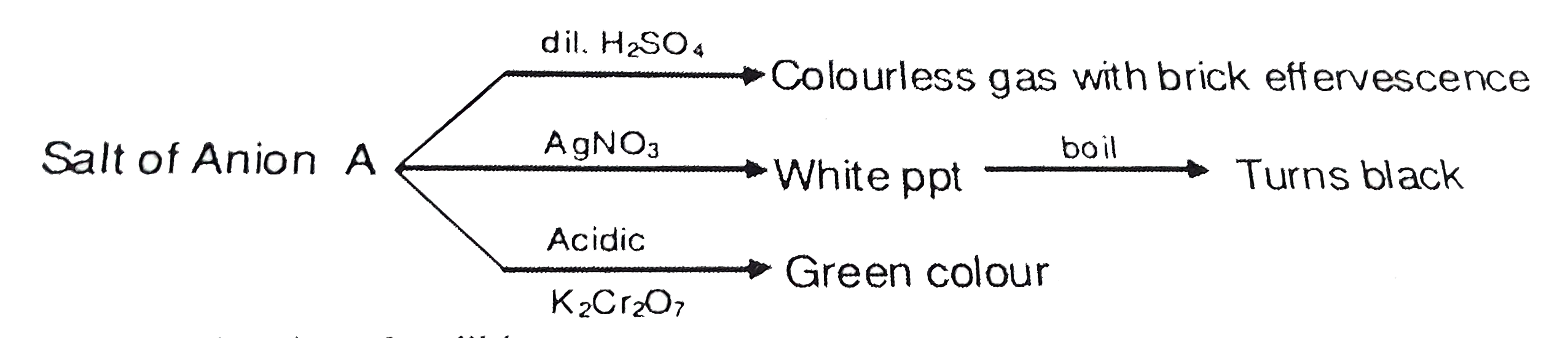
Shape of anion A will be :
Tetrahedral
Trigonal planer
Trigonal pyramidal
Linear
The correct Answer is:C
Step by step video solution for Shape of anion A will be : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Exercise-3
Explore 10 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Additional Problems for Self Practice (APSP)
Explore 30 Videos
- QUALITATIVE ANALYSIS (ANION)Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS (ANION)Exercise:Exercise-1
Explore 58 Videos
- QUALITATIVE ANALYSISBook:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSISExercise:INORGANIC CHMISTRY(Qualitative analysis)
Explore 35 Videos
- QUALITATIVE ANALYSIS PART 1Book:RESONANCE ENGLISHChapter:QUALITATIVE ANALYSIS PART 1Exercise:A.L.P
Explore 37 Videos
Similar Questions
Polarization involves the distortion of the shape of an anion by an adjacently placed cation. In this context, which of the following statements is correct ?
View SolutionAsF5 reacts with XeF4 to form an adduct. The shapes of cation and anion in the adduct are respectively.
View SolutionSbF5 reacts with XeF4 to form an adduct. The shapes of cation and anion in the adduct are respectively :
View SolutionSbF5 reacts with XeF4 to form an adduct. The shapes of cation and anion in the adduct are respectively:
View SolutionStatement-1 : Polarisation is the distortion of the shape of an anion by adjacently placed cation.
and
Statement-2 : Maximum polarisation is brought by a cation of high charge .
View SolutionThe cation/anion radius ratio for a triangular arrangement of anions in which the cation is in contact with the anions (but does not push them apart) as shown in the figure is
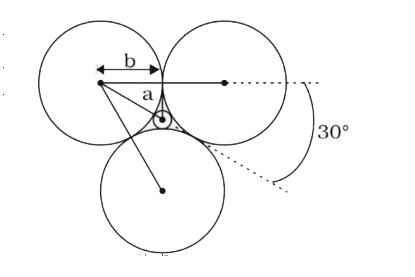
View SolutionWhenever, anion and cation approach each other, the valence shell of the anion is pulled towards the uncleus of the anion is pulled towards the nucles of the cation and as a result, the shape of the anion gets cation is known as polarization of polarizing an amion by a cation is known as polarrization. The ability or capacity of a cation of polarizethe anion towards litsef, is known as its polarizing power. Similiarty. the ability or capacity of an anion to get polarized by the cation, is known as its polarizability. As a result of polarisation, the two ions tend to come closer and the ionic character of the ionic ond acquired partial covalent character. The magnitude of polarization depends upon a number of factors whihc were suggested by Fazan and are termed as Fazan rules.
Which is most covalent in nature ?
View SolutionWhenever, anion and cation approach each other, the valence shell of the anion is pulled towards the uncleus of the anion is pulled towards the nucles of the cation and as a result, the shape of the anion gets cation is known as polarization of polarizing an amion by a cation is known as polarrization. The ability or capacity of a cation of polarizethe anion towards litsef, is known as its polarizing power. Similiarty. the ability or capacity of an anion to get polarized by the cation, is known as its polarizability. As a result of polarisation, the two ions tend to come closer and the ionic character of the ionic ond acquired partial covalent character. The magnitude of polarization depends upon a number of factors whihc were suggested by Fazan and are termed as Fazan rules.
Which has the minimum melting point ?
View SolutionWhen two compounds Acl3andDCl3 of two elements A and D are mixed together a compound ADCl6 is formed. Structural analysis showed that DCl3 is trigonal planar and ACl3 is trigonal pyramidal. If anion has see - saw shape then shape of cation formed is
View SolutionI2 has less solubility in water and its solubility increase on adding KI solution. When KI and I2 react then a species 'X' is formed by which solubility of I2 increases.
Shape of anionic part of 'X' is
View Solution
