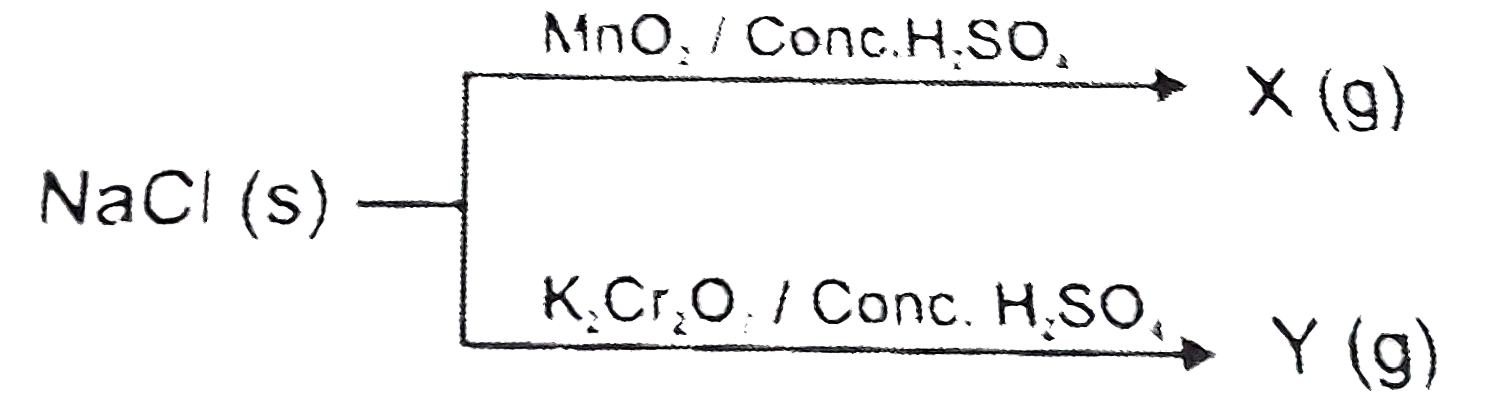
a=difference in the oxidation number of Cl in the product X and product Y, respectively
b= total number of atom in X and Y
c=total number of lone pair in X
then calculate a+b+c = ?
ভিডিও সমাধান
লিখিত জবাব
The correct Answer is:14 (a=1, b=7, c=6)
|
উত্তর
Step by step video solution for a=difference in the oxidation number of Cl in the product X and product Y, respectively b= total number of atom in X and Y c=total number of lone pair in X then calculate a+b+c = ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023টপার এই প্রশ্নগুলো সমাধান করেছে
- QUALITATIVE ANALYSIS (ANION)BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS (ANION)EXERCISE - Exercise-3
10 Videos
- QUALITATIVE ANALYSIS (ANION)BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS (ANION)EXERCISE - Additional Problems for Self Practice (APSP)
30 Videos
- QUALITATIVE ANALYSIS (ANION)BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS (ANION)EXERCISE - Exercise-1
58 Videos
- QUALITATIVE ANALYSISBOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSISEXERCISE - INORGANIC CHMISTRY(Qualitative analysis)
35 Videos
- QUALITATIVE ANALYSIS PART 1BOOK - RESONANCE ENGLISHCHAPTER - QUALITATIVE ANALYSIS PART 1EXERCISE - A.L.P
37 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
The total number of lone pairs of electrons in melamine is
A12B8C10D6Total number of lone pair of electrons in I−3 ion is
A3B6C9D12
The total number of lone pair of electrons in the given molecule is :
View SolutionA total number of lone pairs of electrons in melamine is ….
View SolutionTotal number of lone pair of electrons in XeOF4 is :
View SolutionTotal number of lone pair of electrons in XeOF4 is :
View SolutionThe total number of lone pair of electrons in N2O3 is
View SolutionThe total number of lone-pairs of electrons in melamine is.
View Solution

