[NiCl2{P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic behaviour. The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are:
tetrahedral and tetrahedral
square planar and square planar
tetrahedral and square planar
square planar and tetrahedral
The correct Answer is:C
Step by step video solution for [NiCl_(2){P(C_(2)H_(5))_(2)(C_(6)H_(5))}_(2)] exhibits temperature dependent magnetic behaviour. The coordination geometries of Ni^(2+) in the paramagnetic and diamagnetic states are: by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Exercise-3 Part-I: JEE(Advance ) /IIT-JEE Problem (Comprehenion )
1 Video
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Exercise-3 Part-II: JEE(Main ) /AIEEE Problem
36 Videos
- COORDINATION COMPOUNDSBOOK - RESONANCE ENGLISHCHAPTER - COORDINATION COMPOUNDSEXERCISE - Exercise-2 Part-IV: Comprehension -4
1 Video
- CHEMISTRY IN EVERYDAY LIFEBOOK - RESONANCE ENGLISHCHAPTER - CHEMISTRY IN EVERYDAY LIFEEXERCISE - ORGANIC CHEMISTRY(Chemistry in every day life)
31 Videos
- D & F BLOCK ELEMENTSBOOK - RESONANCE ENGLISHCHAPTER - D & F BLOCK ELEMENTSEXERCISE - ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
15 Videos
Similar Questions
NiCl2KCN−−→complexA
NiCl2KCl−−→excesscomplexB
A & B complexes have the co-ordination number 4.
The IUPAC name of complexes 'A' & 'B' are respectively :
View SolutionOne mole of complex compound Cr(NH3)5CI3 gives 3 moles of ions on dissolution in water One mole of the same complex reacts with two moles of AgNO3 to yield two moles of AgCl The complex is :
View SolutionWhat is gasification ?
View SolutionWhich is the correct statement about birth control pills?
View SolutionThe number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View Solution[NiCI2{P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic behaviour(paramagnetic//diamagnetic) The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively .
View SolutionSelect the correct answer:
a.
i. PhOC2H5 ii. Ph−−−O−−−Ph
iii. PhI iv. C2H5OC2H5
b.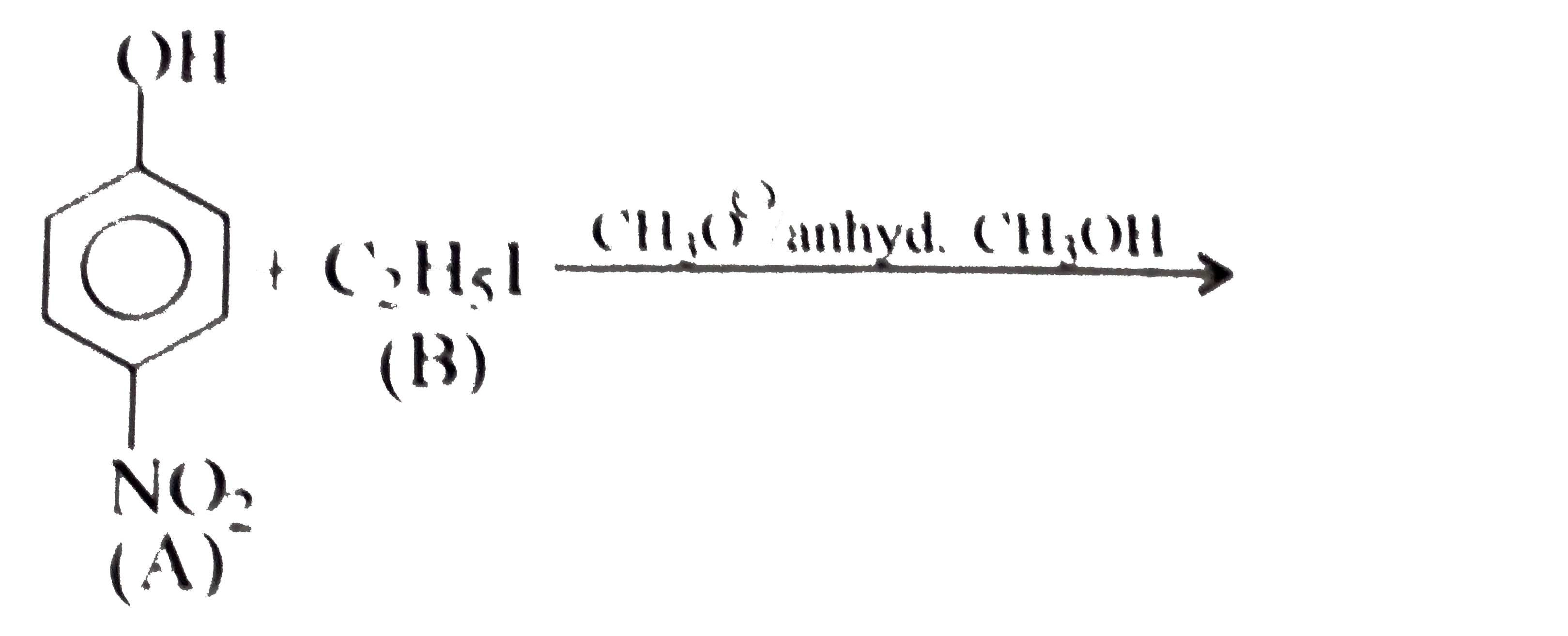
i. p−−NO2−−C6H4−−OC2H5
ii. p−−NO2−−C6H4−−O−−C6H4−−NO2−−p
iii. C2H5−−O−−CH3
iv. p−−NO2−−C6H5−−I.
c.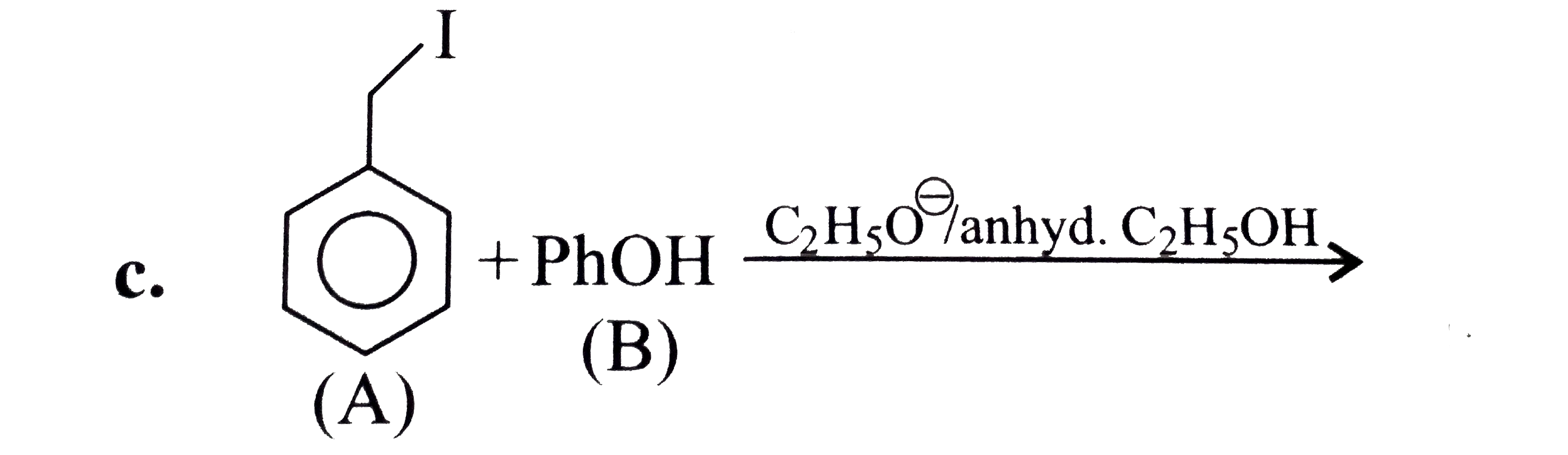
i. C6H5CH2−−O−−Ph
ii. C6H5CH2−−O−−C2H5
iii. Ph−−O−−C2H5
iv. C2H5−−O−−C2H5
d.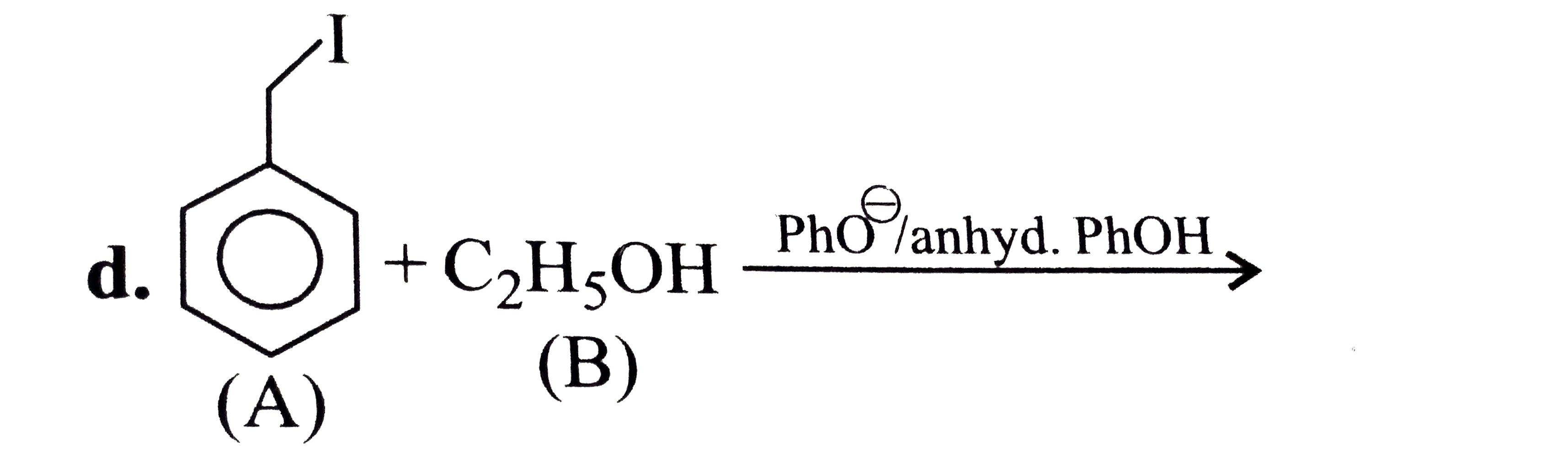
i. C6H5CH2−−O−−C2H5
ii. C6H5−−CH2−−O−−Ph
iii. Ph−−O−−C2H5
iv. C2H5−−O−−C2H5 .
e.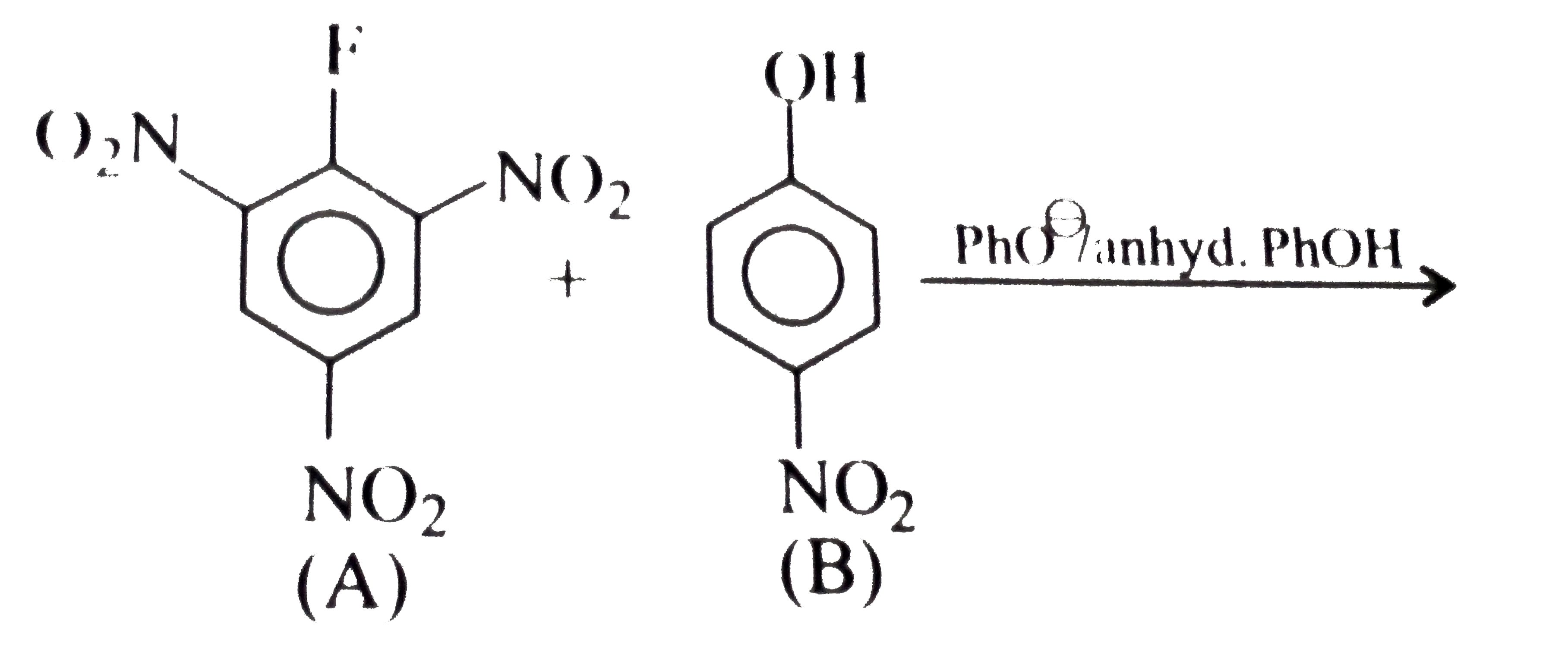
i.
ii.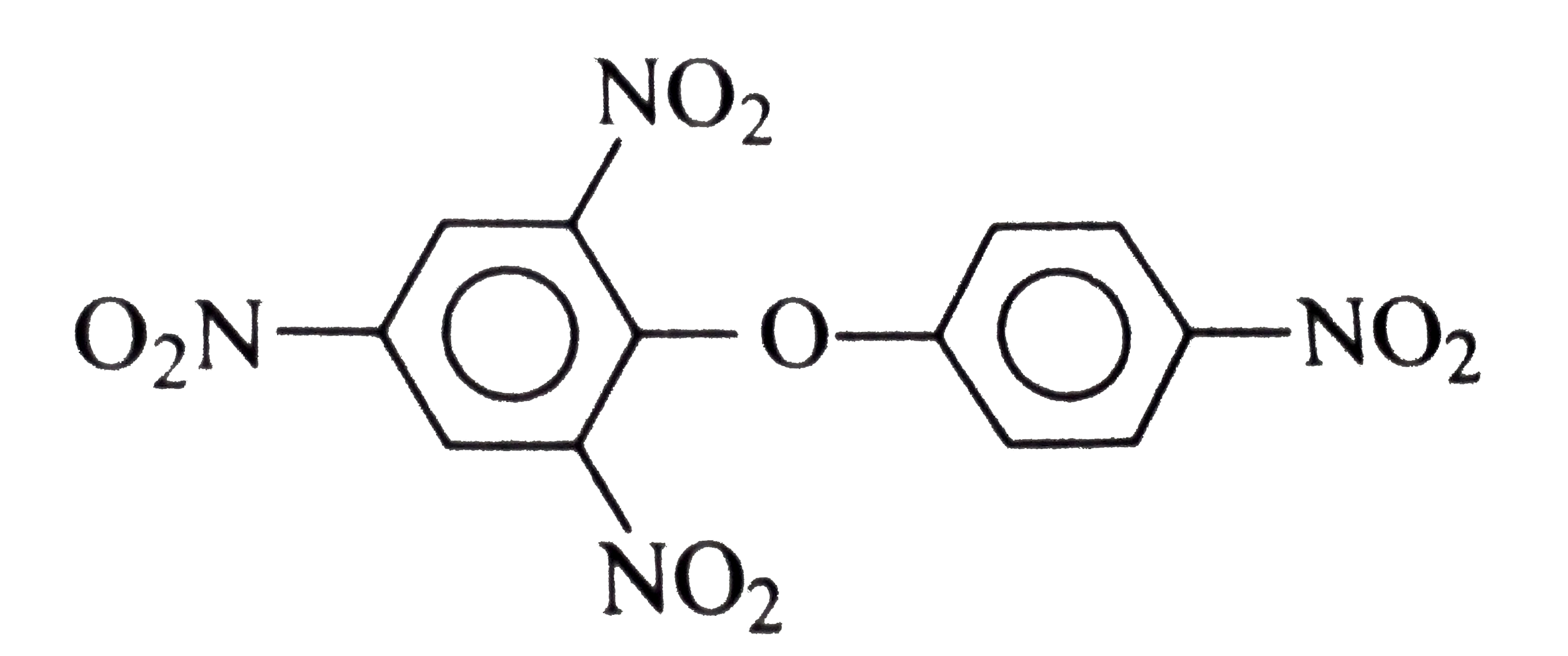
iii.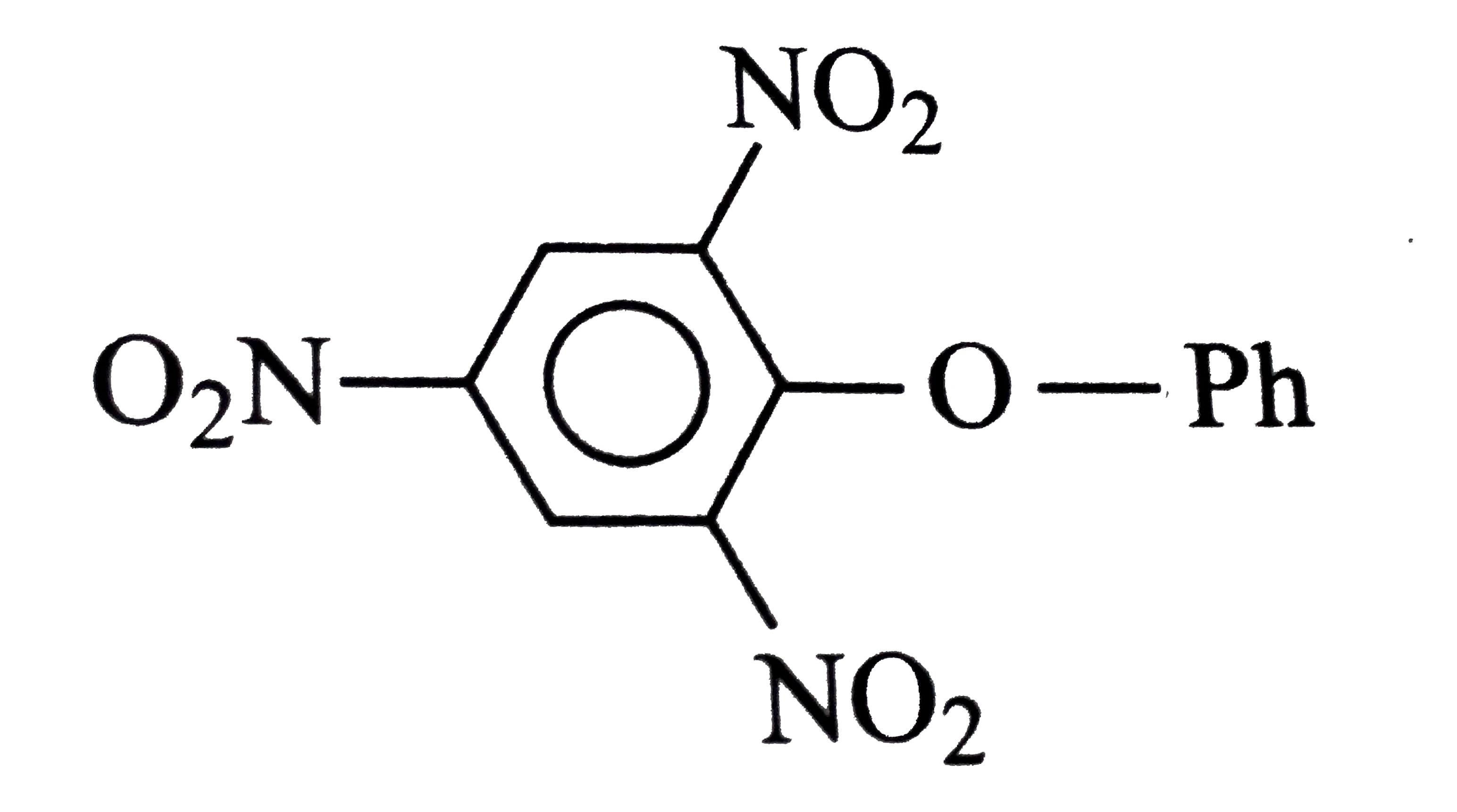
iv.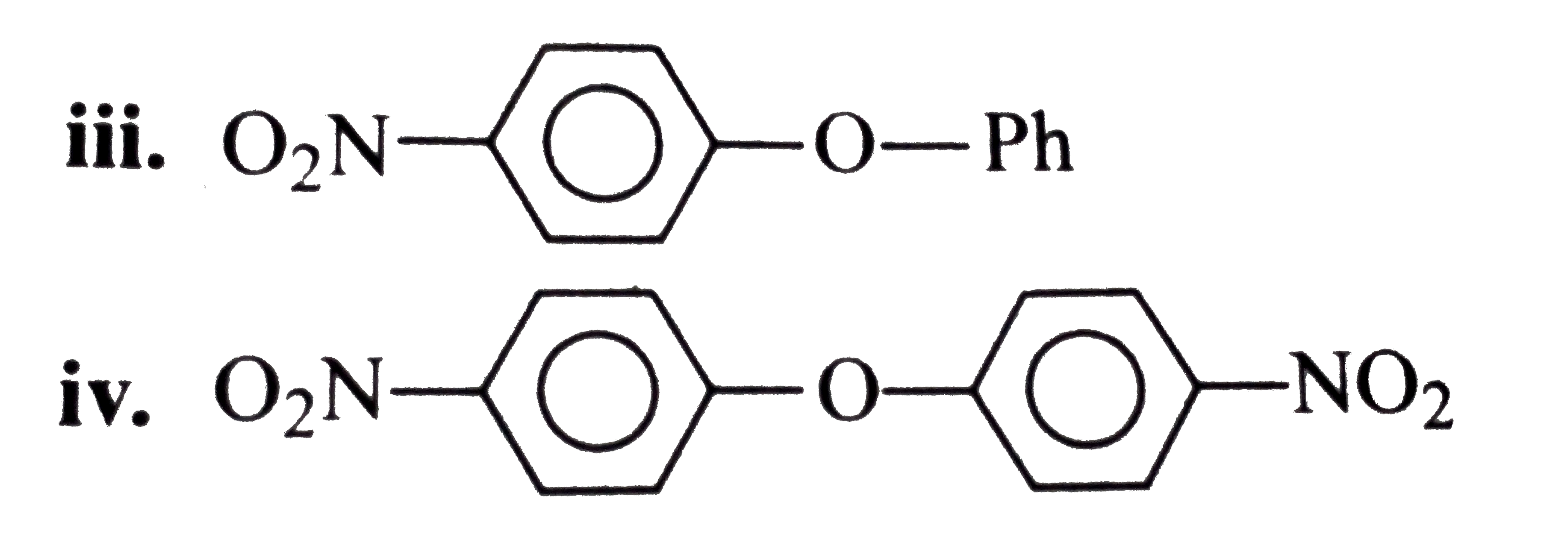
f.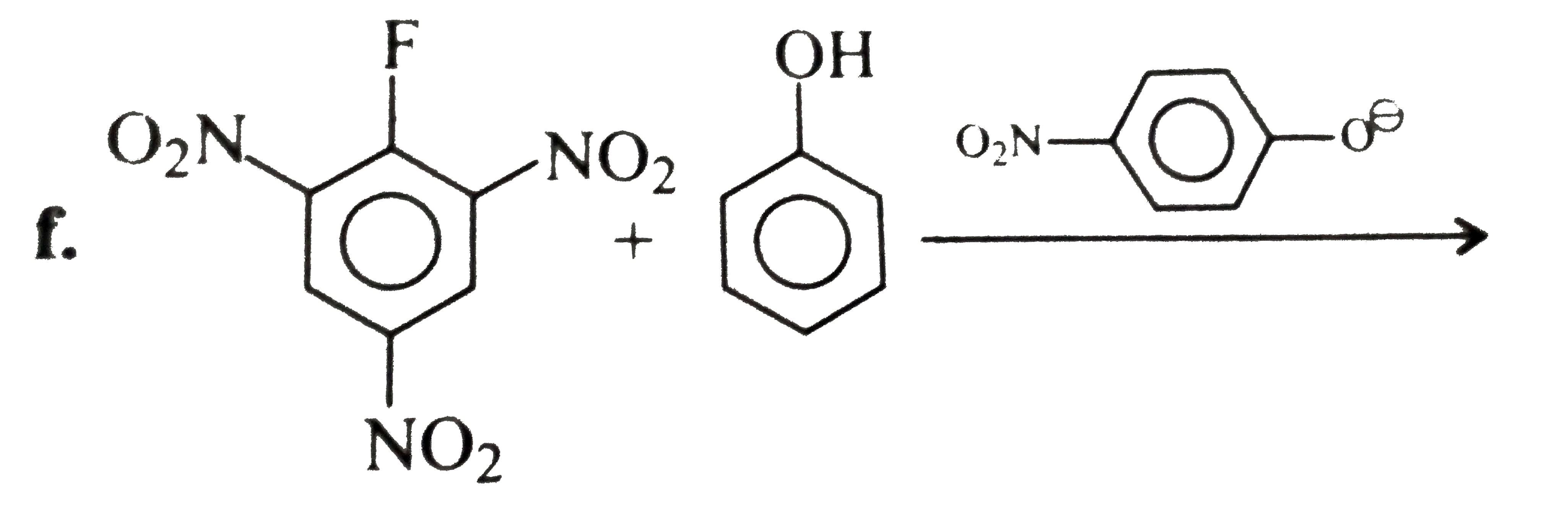
i.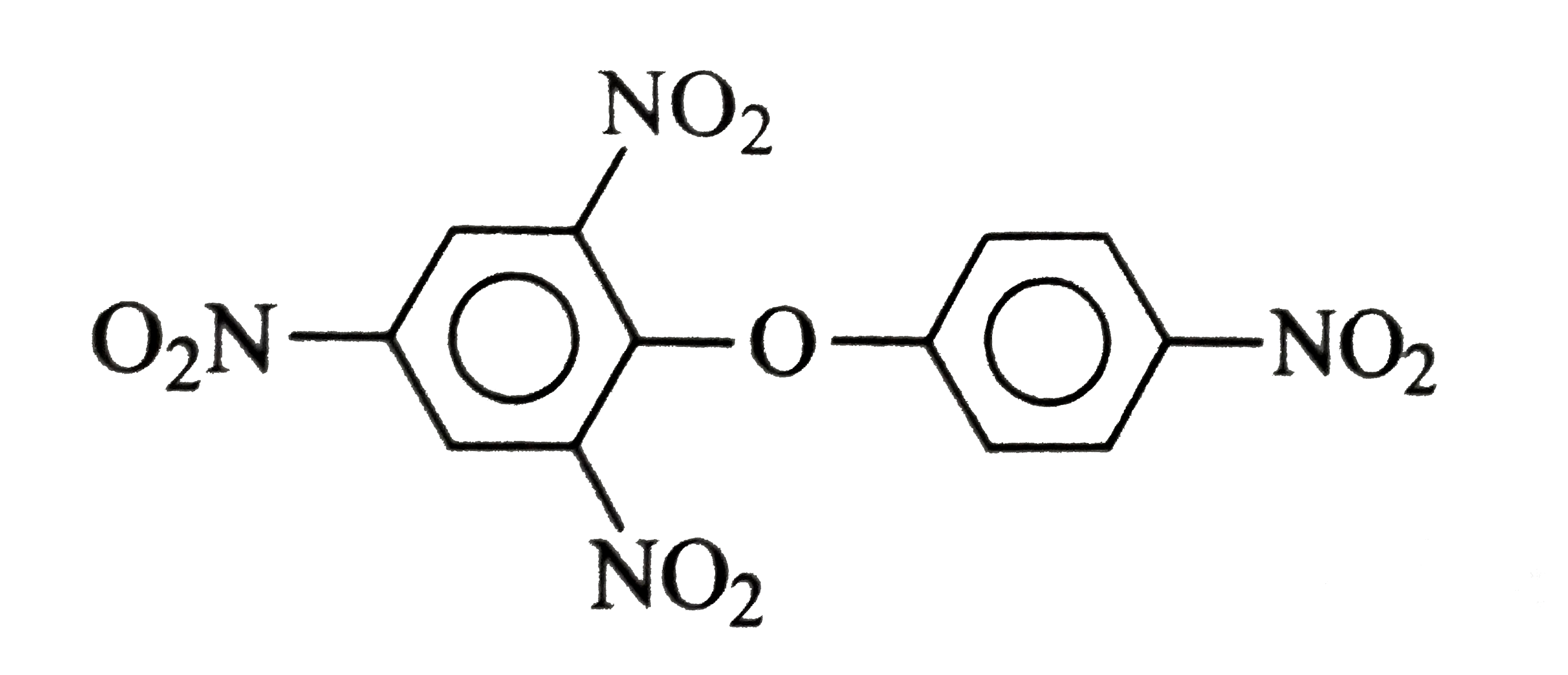
ii.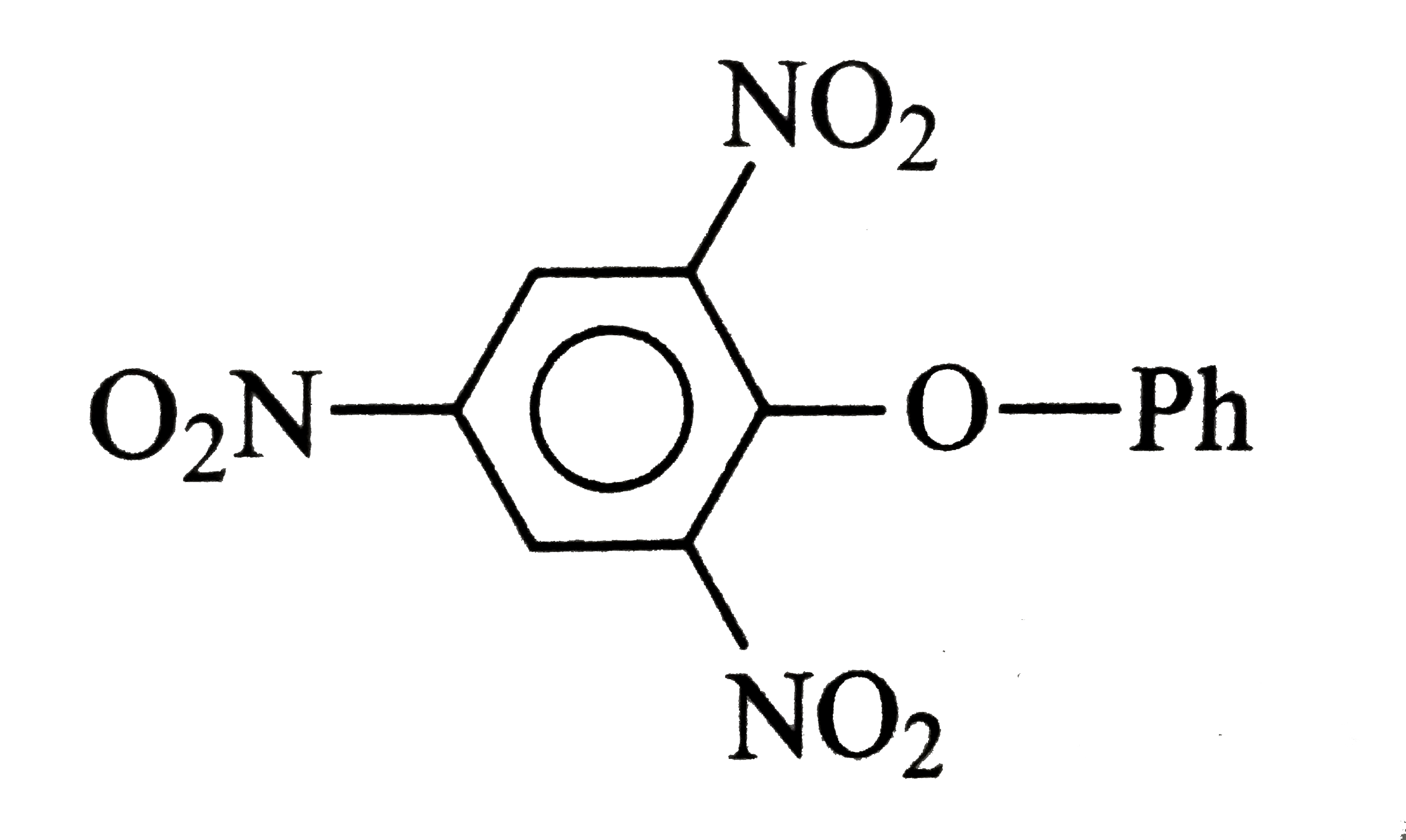
iii.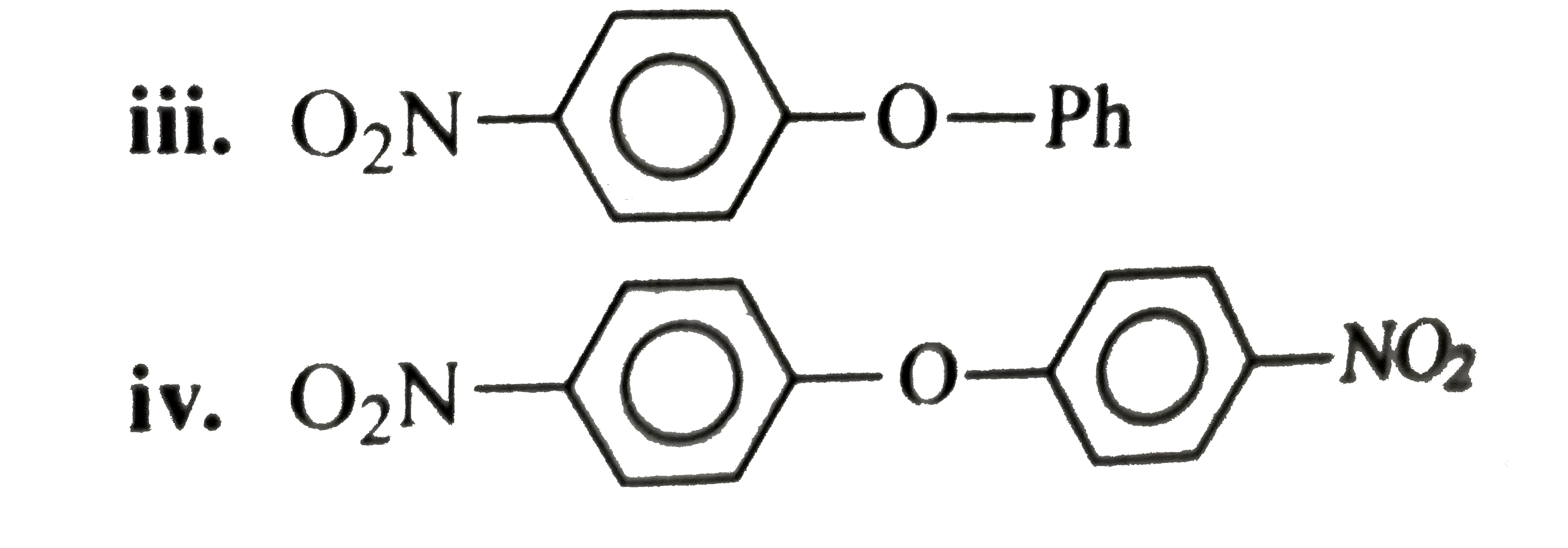
View SolutionArrange the following: (i) In decreasing order of the pK_(b) values: C_(2)H_(5)NH_(2),C_(6)H_(5)NHCH_(3),(C_(2)H_(5))_(2)NHandC_(6)H_(5)NH_(2) (ii) In increasing order of basic strength: C_(6)H_(5)NH_(2),C_(6)H_(5)N(CH_(3))_(2),(C_(2)H_(5))_(2)NH andCH_(3)NH_(2) (iii) In increasing order of basic strength: (a) Aniline, p-nitroaniline and p-toluidine (b) C_(6)H_(5)NH_(2),C_(6)H_(5)NHCH_(3),C_(6)H_(5)CH_(2)NH_(2) (iv) In decreasing order of basic strength in gas phase: C_(2)H_(5)NH_(2),(C_(2)H_(5))_(2)NH,(C_(2)H_(5))_(3)NandNH_(3) (v) In increasing order of boiling point: C_(2)H_(5)OH,(CH_(3))_(2)NH,C_(2)H_(5)NH_(2) (vi) In increasing order of solubility in water: C_(6)H_(5)NH_(2),(C_(2)H_(5))_(2)NH,C_(2)H_(5)NH_(2^.)
View Solutionनिम्नलिखित को क्रम में लिखिए-
(i) pKb मान के घटते क्रम में -
C2H5NH2,C6H5NHCH3,(C2H5)2NH एवं C6H5NH2
(ii) क्षारकीय प्राबल्य के घटते क्रम में-
C6H5NH2,C6H5(CH3)2,(C2H5)2NH एवं C6H5NH2
(iii) क्षारकीय प्राबल्य के बढ़ते क्रम में- (क) ऐनिलीन , पैरा – नाइट्रोऐनिलीन ,एवं पैरा-टॉलूईन
(ख) C6H5NH2,C6H5NHCH3,C6H5CH2NH2
(iv) गैस अवस्था में घटते हुए क्षारकीय प्राबल्य के क्रम में-
C6H5NH2,(C2H5)2NH,(C2H5)3N एवं NH3
(v) क्वथनांक के बढ़ते क्रम में-
C6H5ON,(CH3)2NH,C2H5NH2
(vi) जल में विलेयता के बढ़ते क्रम में-
C6H5OH,(CH3)2NH,C2H5NH2
View SolutionNiCl2[P(C2H5)2(C2H5)]2 ताप आश्रित चुम्बकीय व्यवहार दिखाता है |Ni2+ की अनुचुम्बकीय और प्रतिचुम्बकीय अवस्था में उपसहसंयोजन ज्यमितियाँ क्रमश: है:
View Solutionनिम्नलिखित को क्रम में लिखिएः
(i) pKb मान घटते क्रम में:
C2H5NH2,C6H5NHCH3,(C2H5)2NH एवं C6H5NH2
(ii) क्षारकीय प्राबल्य के घटते क्रम से:
C6NH5NH2,C6H5N(CH3)2,(C2H5)2NH एवं CH3NH2
(iii) क्षारकीय प्राबल्य के बढ़ते क्रम में:
a. ऐनिलीन, पैरा-नाइइट्रोऐनिलीन एवं पैरा-टॉलूडीन
b. C6H5NH2,C6H5NHCHH3,C6H5C2NH2
(iv) गैस अवस्था में घटते हुए क्षारकीय प्राबलय के क्रम में: C2H5NH2,(C2H5)2NH,(C2H5)3N एवं NH3
(v) क्वथनांक के बढ़ते क्रम में
C2H5OH,(CH3)2,NH,C2H5NH2
(vi) जल में विलेयता के बढ़ते क्रम में:
C6H5NH2,(C2H4)2NH,C2H5NH2
View Solutionनिम्न को निर्देशानुसार कीजिए-
(i)pKb मानो के घटते क्रम में-
C2H5NH2, C6H5NHCH3, (C2H5)2NH C6H5NH2
(ii) क्षारकीयता बढ़ते क्रम में-
C6H5NH2, C6H5N(CH3)2, (C2H5)2andCH3NH2
(iii) क्षारकीयता के बढ़ते क्रम में-
(a) Antiline, p-nitroaniline and p-toluidine
(b) C6H5NH2,C6H5NHCH3,C6H5CH2NH2
(iv) गैस प्रावस्था में क्षारकीयता के घटते क्रम में-
C2H5NH2,(C2H5)2NH,(C2H5)3NandNH3
(v) क्वथनांक के बढ़ते क्रम में-
C2H5OH,(CH3)2NH,C2H5NH2
(vi) जल में वलयन के बढ़ते क्रम में-
C6H5NH2,(C2H5)2NH,C2H5NH2.
View Solutionनिम्नलिखित को क्रम में लिखिए -
(i) pKb मान के घटते क्रम में -
C2H5NH2,C6H5NHCH3,(C2H5)2NHएवं C6H5NH2
(ii) क्षारकीय प्राबल्य के घटते क्रम में -
C6H5NH2,C6H5N(CH3)2,(C2H5)2NH एवं CH3NH2
(iii) क्षारकीय प्राबल्य के बढ़ते क्रम में -
(a) ऐनिलीन , पैरा - नाइट्रोऐनिलीन एवं पैरा-टोलूडीन
(b) C6H5NH2,C6H5NHCH3,C6H5CH2NH2
(iv) गैस अवस्था में घटते हुए क्षारकीय प्राबल्य के क्रम में -
C2H5OH,(C2H5)2NH,(C2H5)3N एवं NH3
(v) क्वथनांक के बढ़ते क्रम में -
C2H5OH,(CH3)2NH,C2H5NH2
(vi) जल में विलेयता के बढ़ते क्रम में -
C6H5NH2,(C2H5)2NH,C2H5NH2.
View Solution[NiCl2{P(C2H5)2(C6H5)]2] exibits temperature dependent magnetic behaviour (paramagnetic/diamangetic). The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively
View Solution[NiCl2{P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic behaviour. The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are:
View SolutionNiCl2[P(C2H5)2(C2H5)]2 ताप आश्रित चुम्बकीय व्यवहार दिखाता है |Ni2+ की अनुचुम्बकीय और प्रतिचुम्बकीय अवस्था में उपसहसंयोजन ज्यमितियाँ क्रमश: है:
View Solution

