The magnetic moment for two complexes of empirical formula Ni(NH3)4(NO3)2.2H2O is zero and 2.84 BM respectively. The second complex is not a netural complex.
The number of water molecules of crystallization are respectively
zero, two
zero, zero
two, zero
two ,two
The correct Answer is:C
To solve the problem regarding the magnetic moments of the two complexes of empirical formula Ni(NH₃)₄(NO₃)₂·2H₂O, we will analyze the information given step by step.
Step 1: Analyze the first complex
The first complex has a magnetic moment (μ) of 0 BM.
- Magnetic moment formula:
μ=√n(n+2)
where n is the number of unpaired electrons.
- Since μ = 0, we can conclude that:
n(n+2)=0⟹n=0
This means that there are no unpaired electrons in the first complex.
Step 2: Determine the oxidation state of Nickel in the first complex
In the first complex, we have:
- Ligands: 4 NH₃ (neutral) and 2 NO₃⁻ (each with a charge of -1).
- Let the oxidation state of Nickel (Ni) be x.
The overall charge of the complex is neutral (0):
x+0+(−2)=0⟹x=+2
Thus, Nickel is in the +2 oxidation state.
Step 3: Analyze the second complex
The second complex has a magnetic moment (μ) of 2.84 BM.
- Using the magnetic moment formula again:
2.84=√n(n+2)
Squaring both sides:
2.842=n(n+2)⟹8.0656=n(n+2)
This can be approximated to:
n(n+2)≈8⟹n2+2n−8=0
Solving this quadratic equation using the quadratic formula:
n=−2±√4+322=−2±62
This gives us:
n=2(since n must be non-negative)
Thus, there are 2 unpaired electrons in the second complex.
Step 4: Determine the oxidation state of Nickel in the second complex
The second complex is not neutral, which means it has a net charge.
Assuming the same ligands:
- 4 NH₃ (neutral) and 2 NO₃⁻ (each with a charge of -1).
- The oxidation state of Nickel is still x.
Let’s denote the charge of the second complex as +1 (since it is not neutral):
x+0+(−2)=+1⟹x−2=1⟹x=+3
Thus, Nickel is in the +3 oxidation state in the second complex.
Step 5: Identify the number of water molecules of crystallization
- In the first complex, the 2 water molecules (2H₂O) are outside the coordination sphere and are considered as water of crystallization.
- In the second complex, the 2 water molecules are not mentioned to be outside the coordination sphere, which means they are likely part of the coordination sphere and thus not counted as water of crystallization.
Conclusion
The number of water molecules of crystallization for the two complexes are:
- First complex: 2
- Second complex: 0
Final Answer
The number of water molecules of crystallization are respectively 2 and 0.
---
The first complex has a magnetic moment (μ) of 0 BM.
μ=√n(n+2)
where n is the number of unpaired electrons.
n(n+2)=0⟹n=0
This means that there are no unpaired electrons in the first complex.
In the first complex, we have:
- Ligands: 4 NH₃ (neutral) and 2 NO₃⁻ (each with a charge of -1).
- Let the oxidation state of Nickel (Ni) be x.
x+0+(−2)=0⟹x=+2
Thus, Nickel is in the +2 oxidation state.
The second complex has a magnetic moment (μ) of 2.84 BM.
2.84=√n(n+2)
Squaring both sides:
2.842=n(n+2)⟹8.0656=n(n+2)
This can be approximated to:
n(n+2)≈8⟹n2+2n−8=0
Solving this quadratic equation using the quadratic formula:
n=−2±√4+322=−2±62
This gives us:
n=2(since n must be non-negative)
Thus, there are 2 unpaired electrons in the second complex.
The second complex is not neutral, which means it has a net charge.
- 4 NH₃ (neutral) and 2 NO₃⁻ (each with a charge of -1).
- The oxidation state of Nickel is still x.
x+0+(−2)=+1⟹x−2=1⟹x=+3
Thus, Nickel is in the +3 oxidation state in the second complex.
- In the first complex, the 2 water molecules (2H₂O) are outside the coordination sphere and are considered as water of crystallization.
- In the second complex, the 2 water molecules are not mentioned to be outside the coordination sphere, which means they are likely part of the coordination sphere and thus not counted as water of crystallization.
The number of water molecules of crystallization for the two complexes are:
- First complex: 2
- Second complex: 0
The number of water molecules of crystallization are respectively 2 and 0.
Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:RESONANCE ENGLISHChapter:COORDINATION COMPOUNDSExercise:Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-5) (Matching Lift type )
Explore 1 Video
- COORDINATION COMPOUNDSBook:RESONANCE ENGLISHChapter:COORDINATION COMPOUNDSExercise:Additional Problem for Self Practice (APSP) Part-IV Practice Test -2 (Section-3) (One Integer Value Correct Type )
Explore 5 Videos
- CHEMISTRY IN EVERYDAY LIFEBook:RESONANCE ENGLISHChapter:CHEMISTRY IN EVERYDAY LIFEExercise:ORGANIC CHEMISTRY(Chemistry in every day life)
Explore 31 Videos
- D & F BLOCK ELEMENTSBook:RESONANCE ENGLISHChapter:D & F BLOCK ELEMENTSExercise:ORGANIC CHEMISTRY(Aldehydes , Ketones, Carboxylic acid)
Explore 15 Videos
Similar Questions
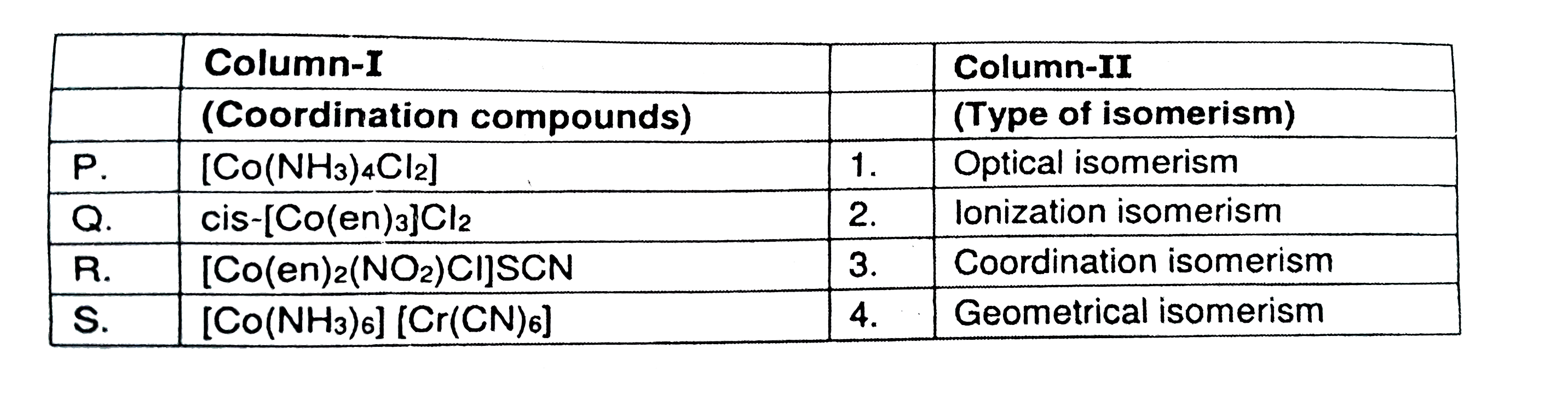
Column-I and Column-II contains four entries each. Entries of Column-I are to be matched with some entries of Column-II One or more than one entries of Column-I may have the matching with the same entries of Column-II.
View SolutionIn the complex Fe(CO)x, the value of x is if its EAN is 36.
View SolutionWhich is the correct statement about birth control pills?
View SolutionThe number of photons of light of ¯v=3.5×106m−1 necessary to provide 2 J of energy are:
View SolutionThe total number of isomer shown by [Co(NH_(3))_(4)(NO_(2))_(2)](NO_(3)) complex is :
View SolutionThe magentic momentsn(BM) of the complexes [Ni(H_(2)O)_(6)]^(2+)" and "[Ni(NH_(3))_(6)]^(2+) are resectively
View Solutionunderset("Brown")([(NH_(3))_(5)Co-O-O-Co(NH_(3))_(5)]^(4+)) underset("oxidise")overset([S_(2)O_(8)]^(2-))tounderset("Green")([(NH_(3))_(5)Co-O-O-Co(NH_(3))_(5)]^(+5)) The magnetic moment of green complex is 1.7 BM & for brown complexes magnetic moment is zero. (O-O) is same in all respect in both the complexes. The O.S. of Co in brown complex & green complex respectively are-
View SolutionThe magnetic moment for two complexes of empirical formula Ni(NH_(3))_(4)(NO_(3))_(2).2H_(2)O is zero and 2.84 BM respectively. The second complex is not a netural complex. The number of water molecules of crystallization are respectively
View SolutionThe magnetic moment for two complexes of empirical formula Ni(NH_(3))_(4)(NO_(3))_(2).2H_(2)O is zero and 2.84 BM respectively. The second complex is not a netural complex. The correct formula and geometry of the first complex is :
View SolutionThe magnetic moment for two complexes of empirical formula Ni(NH_(3))_(4)(NO_(3))_(2).2H_(2)O is zero and 2.84 BM respectively. The second complex is not a netural complex. Which of the following statement are true for the second complex ?
View Solution{:([(NH_(3))_(5)Co-O-O-Co(NH_(3))_(5)]^(4+)underset("oxidise") overset([S_(2)O_(8)]^(2-))to [(NH_(3))_(5)Co-O-O-Co(NH_(3))_(5)]^(5+)),(" ""(Brown)"" ""(Green)"):} The magnetic moment of green complex is 1.7 BM and for brown complexes magnetic moment is zero. (O-O) is same in all respect in both the complexes. The oxidation state of Co in brown complex and green complex respectively are :
View Solution[(NH3)5Co−O−O−Co(NH3)5]4+[S2O8]2−−−−−−→oxidise[(NH3)5Co−O−O−Co(NH3)5]5+ (Brown) (Green)
The magnetic moment of green complex is 1.7 BM and for brown complexes magnetic moment is zero. (O−O) is same in all respect in both the complexes. The oxidation state of Co in brown complex and green complex respectively are :
View Solution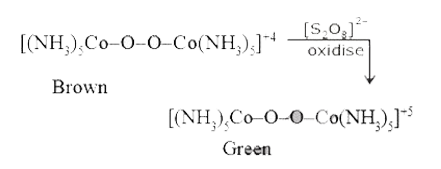
The magnetic moment of green complex is 1.7 BM & for brown complexes magnetic moment is zero. (O–O) is same in all respect in both the complexes. The O. S. of Co in brown complex & green complex respectively are–
View SolutionThe hexaaqua complex[Ni(H2O)6]2+ is green, whereas the corresponding ammonia complex [Ni(NH3)6]2+ is violet. Explain.
View SolutionThe hybridization of 'Cr' in the complex [Cr(NO_(2))_(4)(NH_(3))_(2)]^(-)
View SolutionThe coordination number and magnetic moment of the complex [Cr(C2O4)2(NH3)2]−Jet respectively is
View Solution
