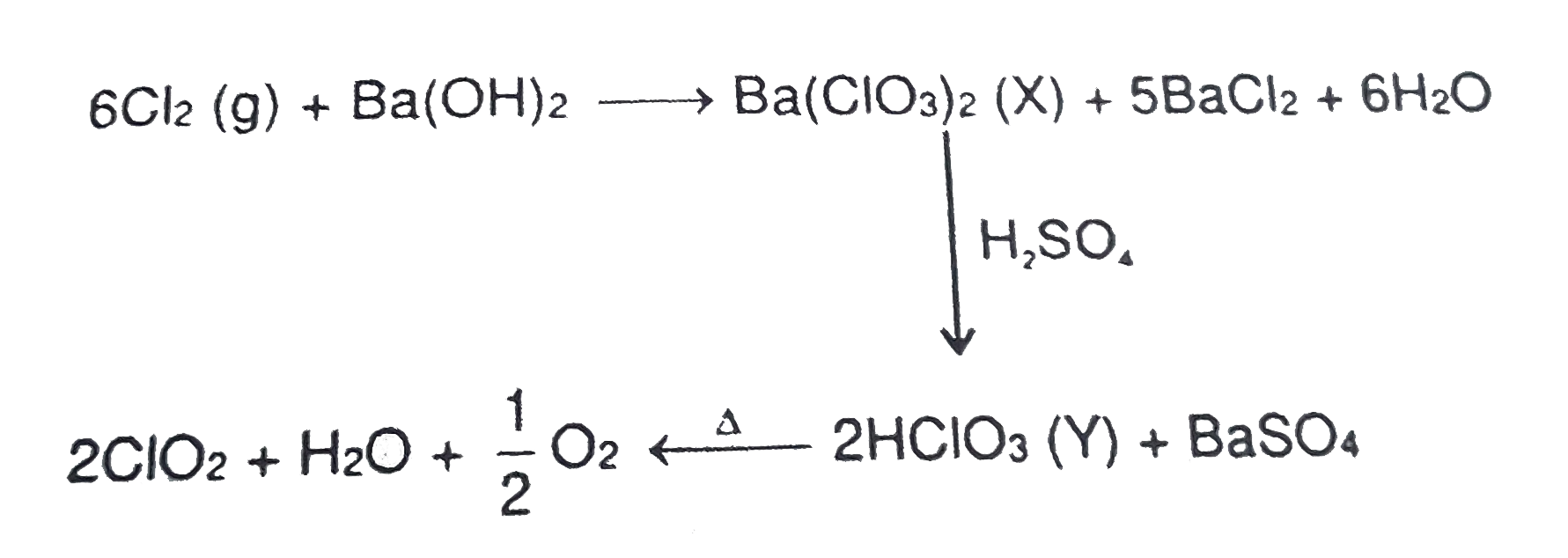Cl2(g)+Ba(OH)2→X(aq.)+BaCl2+H2O
X+H2SO4→Y+BaSO4
YΔ−−−−−→Δ>365KZ+H2O+O2
Y and Z are respectively :
A
HClO4,CIO2
B
HClO3,ClO2
C
HClO3,ClO6
D
HClO4,Cl2O7
Video Solution
Text Solution
The correct Answer is:B
|
Topper's Solved these Questions
- P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Exercise:APSP PART-2
Explore 21 Videos
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Exercise:PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
Explore 8 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Advanced Level Problems
Explore 105 Videos
Similar Questions
Knowledge Check
Na2B4O7.10H2OΔ−−→XΔ−−→Y+Z,XY and Z in the reaction are
AX=Na2B4O7,YNaBO2,Z=B2O3BX=Na2B4O7,Y=B2O3,Z=H3BO3CX=B2O3,Y=NaBO2,Z=B(OH)3DX=NaBO2,Y=B2O3,Z=B(OH)3
Ba(OH)2+SO2→BaSO3⏐↓+H2O
View SolutionMg(s)+C(s)Δ−−→highTXH2O−−→Mg(OH)2+Y
X and Y are respectively:
View SolutionS––O2Cl2+H2O→H2SO4+HCl
View SolutionCH3CH2COONH4P2O5−−→ΔXH+/H2O−−−−−→Y.
Y and X respectively are
View SolutionNH4NO3Δ−−→N2O+H2O
View SolutionNH4NO3Δ−−→N2O+H2O
View SolutionNH3+O2Pt−−→ΔA+H2O
A+O2→B,
B+O2+H2O→C
A, B and C are:
View Solution