The simplest ratio x:y of xenon and fluorine when passes through Ni-tube (400∘C) at high pressure gives XeF6. Here x+y is :
The correct Answer is:21
To find the simplest ratio x:y of xenon (Xe) to fluorine (F2) that produces xenon hexafluoride (XeF6) when passed through a nickel tube at 400°C and high pressure, we can follow these steps:
Step 1: Understand the Reaction
The chemical reaction for the formation of xenon hexafluoride can be represented as:
Xe+F2→XeF6
Step 2: Determine the Stoichiometry
From the chemical formula of xenon hexafluoride, we see that one mole of xenon reacts with six moles of fluorine:
1 Xe+6 F2→1 XeF6
Step 3: Establish the Ratio
To find the simplest ratio x:y of xenon to fluorine, we can express it in terms of moles:
- For every 1 mole of xenon (Xe), we need 6 moles of fluorine (F2).
- Therefore, the ratio x:y can be written as:
x:y=1:6
Step 4: Calculate x+y
Now, we need to find x+y:
- Here, x=1 and y=6.
- Thus, x+y=1+6=7.
Final Answer
The value of x+y is:
7
The chemical reaction for the formation of xenon hexafluoride can be represented as:
Xe+F2→XeF6
From the chemical formula of xenon hexafluoride, we see that one mole of xenon reacts with six moles of fluorine:
1 Xe+6 F2→1 XeF6
To find the simplest ratio x:y of xenon to fluorine, we can express it in terms of moles:
- For every 1 mole of xenon (Xe), we need 6 moles of fluorine (F2).
- Therefore, the ratio x:y can be written as:
x:y=1:6
Now, we need to find x+y:
- Here, x=1 and y=6.
- Thus, x+y=1+6=7.
The value of x+y is:
7
Topper's Solved these Questions
- P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)Exercise:APSP PART-2
Explore 21 Videos
- P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Book:RESONANCE ENGLISHChapter:P-BLOCK ELEMENT (BORON AND CARBON FAMILY)Exercise:PART - III : OLYMPIAD PROBLEMS (PREVIOUS YEARS) STAGE - V (INTERNATIONAL CHEMISTRY OLYMPIAD (IChO)) Problem 3
Explore 8 Videos
- PERIODIC TABLE & PERIODICITYBook:RESONANCE ENGLISHChapter:PERIODIC TABLE & PERIODICITYExercise:Advanced Level Problems
Explore 105 Videos
Similar Questions
Acid used for making permanent marking on the glass surface is:
View SolutionGive examples of the silicate minerals :
feldspar , zeolites , micas , asbestos ?
View SolutionThe elements which exhibit both vertical and horizontal similarities are:
View SolutionPropyne when passed through a hot iron tube at 400∘C produces
View SolutionThe simplest ratio x:y of xenon and fluorine when passes through Ni-tube (400^@C) at high pressure gives XeF_6 . Here x+y is :
View SolutionWhen ethene is heated at 400^@ C Under high pressure, the product is are
View SolutionXenon reacts with fluorine at 873 K and 7 bar to form XeF_(4) . In this reaction, the ratio of xenon and fluorine required is
View Solutionजब एथीन को उच्च दाब पर 400∘C पर गर्म किया जाये तो प्राप्त होता है
View SolutionIn XeF6 molecule, Xenon atom undergoes
View SolutionIn XeF6 molecule, Xenon atom undergoes
View SolutionXenon Fluorides : Xenon reacts directly with fluorine on heating at 675 K in a sealed nickel tube. The product obtained depends upon the amount of fluorine present.
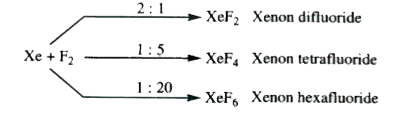
XeF6 on complete hydrolysis gives
View SolutionPropyen when passed through a hot iron tube at 400^(@)C produces
View SolutionWhat happens when xenon and fluorine are reacted in the ratio 1:5 respectively?
View SolutionWhat happens when xenon and fluorine are reacted in the ratio 1:20 respectively?
View SolutionPropyne when passed through a hot iron tube at 400∘C produces
View Solution
